Esters
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Uses
Have characteristic sweet smells used as food flavouring and perfumes
Used as plasticiers but will ‘escape’ over time leaving the plastic stiff and brittle
Used as solvents for polar organic molecules
Hydrolysis of esters
Will undergo hydrolysis in acid or alkaline solution
Hot and alkaline is preferred as acid conditions makes the reaction reversible + slow
Alkaline Hydrolysis - products and use
Leads to formation of sodium salt of the carboxylic acid and alcohol
e.g. RCOOR + NaOH → RCOONA + ROH
If excess H2SO4 or HCl is added to sodium salt then carboxylic acid reforms
e.g. HCOONA + HCl → HCOOH + NaCl
Sodium salts of long chain carboxylic acids is used in soaps
Acid Hydrolysis
Reversal of esterification
e.g. CH3CH2COOCH3 + H2O → CH3CH2COOH + CH3OH
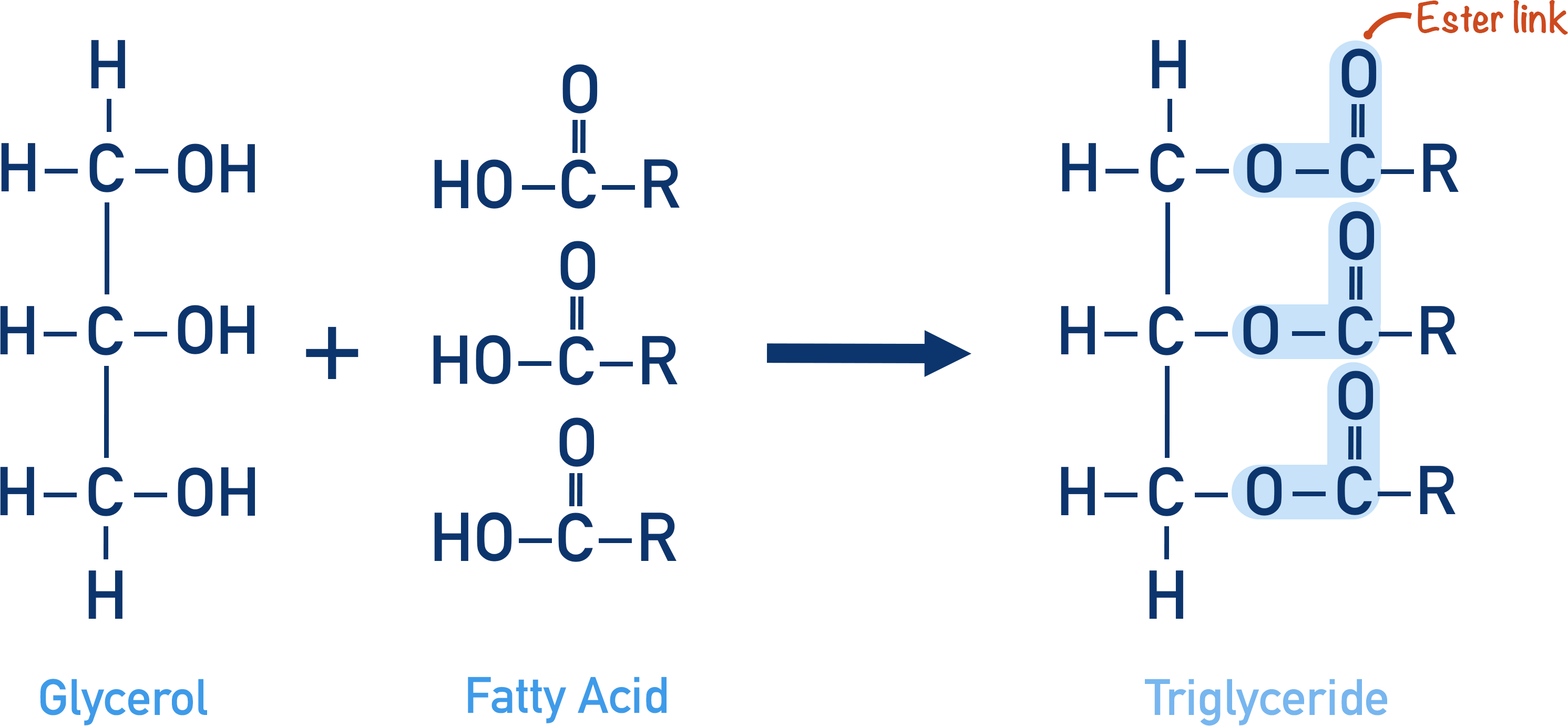
Triglycerides
Triesters of fatty acids and glycerol
Occur naturally as fats and oils
Fats are soldies at room temperatures and contain saturated acids
Oils are liquids at room temperature and show some degree of unsaturation in the acid
Formation of triglyceride
Alkaline Hydrolysis of triesters
Leads to formation of glycerol and sodium salts of the long chain carboxylic acids
Used to make soap
Acid Hydrolysis of triesters
Forms glycerol and the carboxylic acids
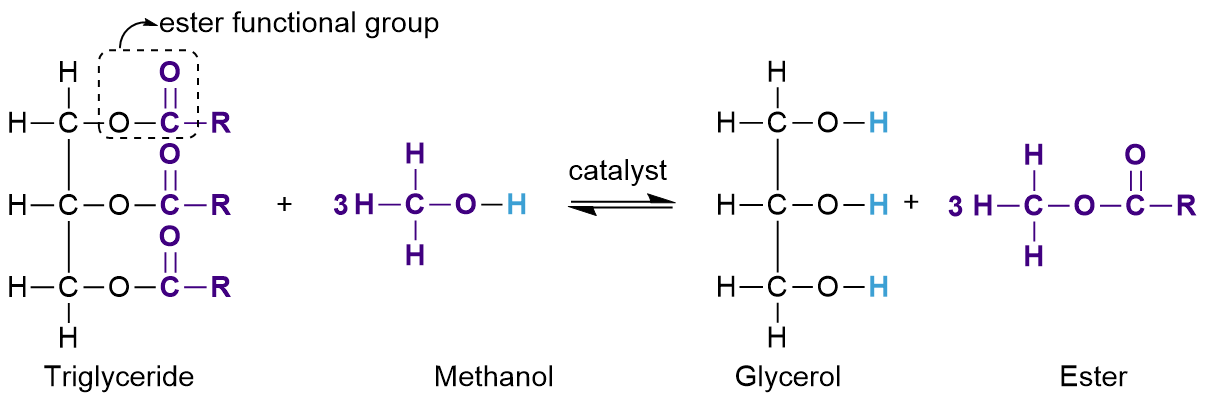
Biodiesel
Alternative, renewable fuel made from oil obtained from crops such as rapeseed oil
Can be converted into biodiesel by transesterification by using methanol
Why only methyl esters of long chain acids used as biodiesel?
They are non-toxic
Biodegradable
Burns cleaner than petrol
Cheaper than ordinary diesel fuel