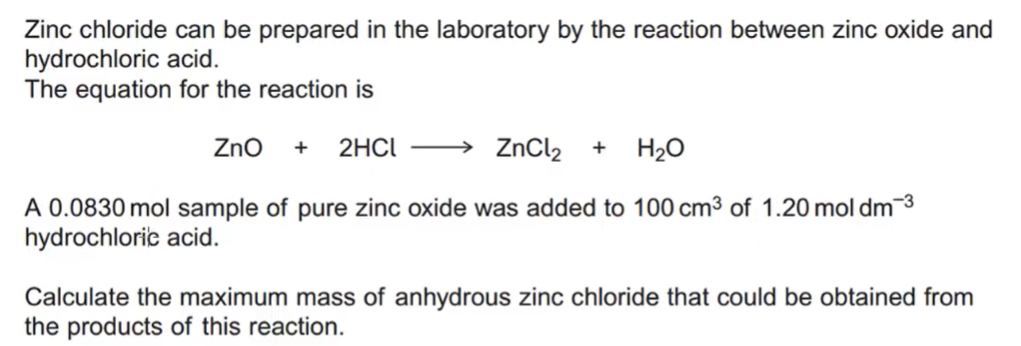
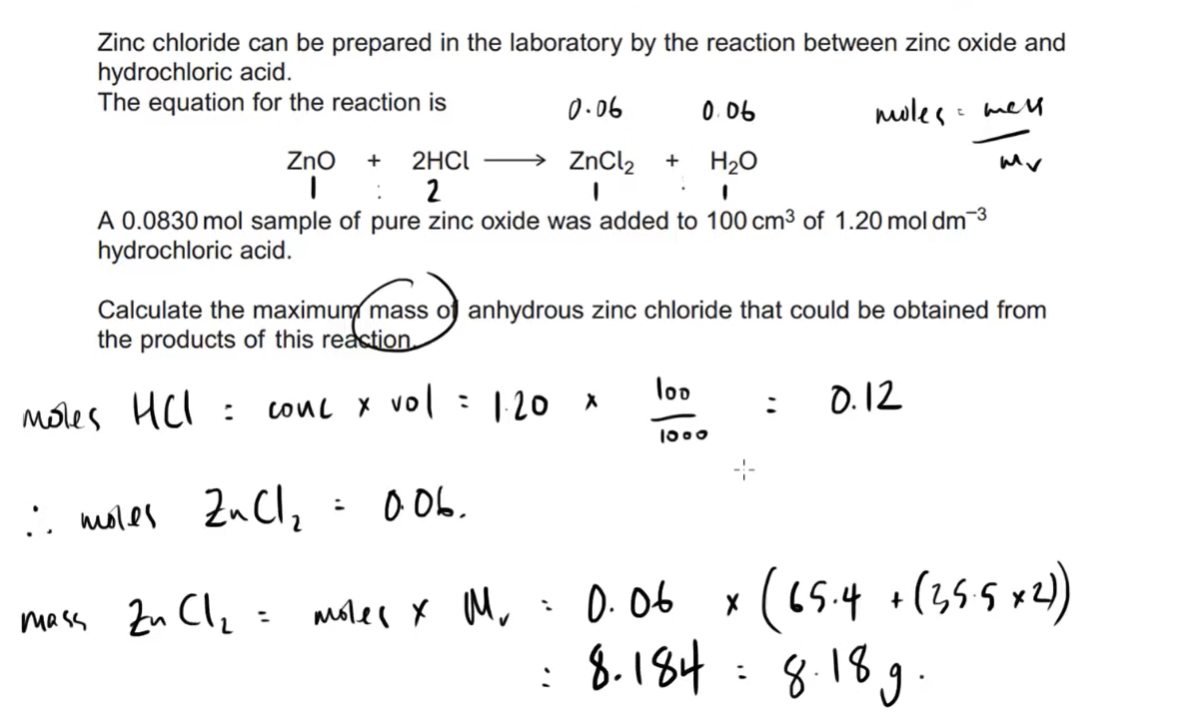
the mole and the avogadro constant
0.0(0)
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
9 Terms
1
New cards
what is a mole?
The mole is the amount of substance in grams that has the same number of particles as there are atoms in 12 grams of carbon-12.
(like a word that describes a number like a dozen=12, a mole= 6.023 X 1023)
2
New cards
1st calculation to calculate moles?
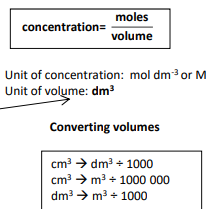
3
New cards
2nd?
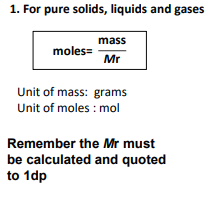
4
New cards
what does one mole of an atom and molecule =
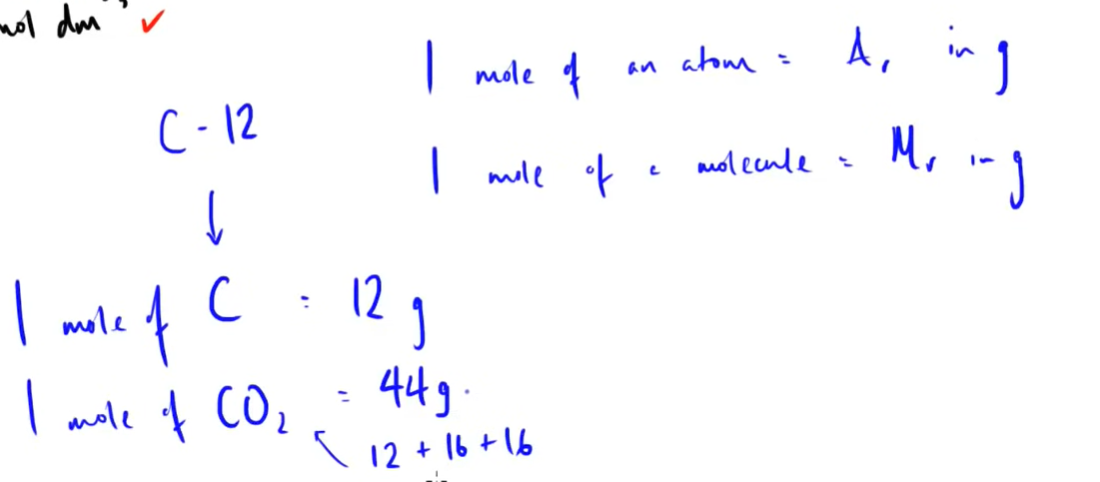
5
New cards
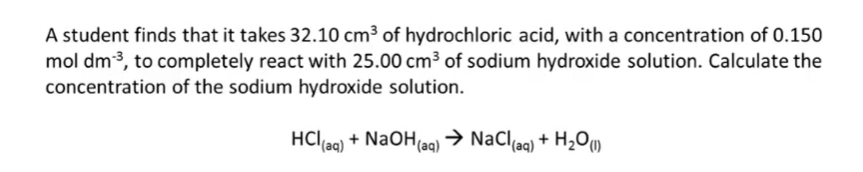
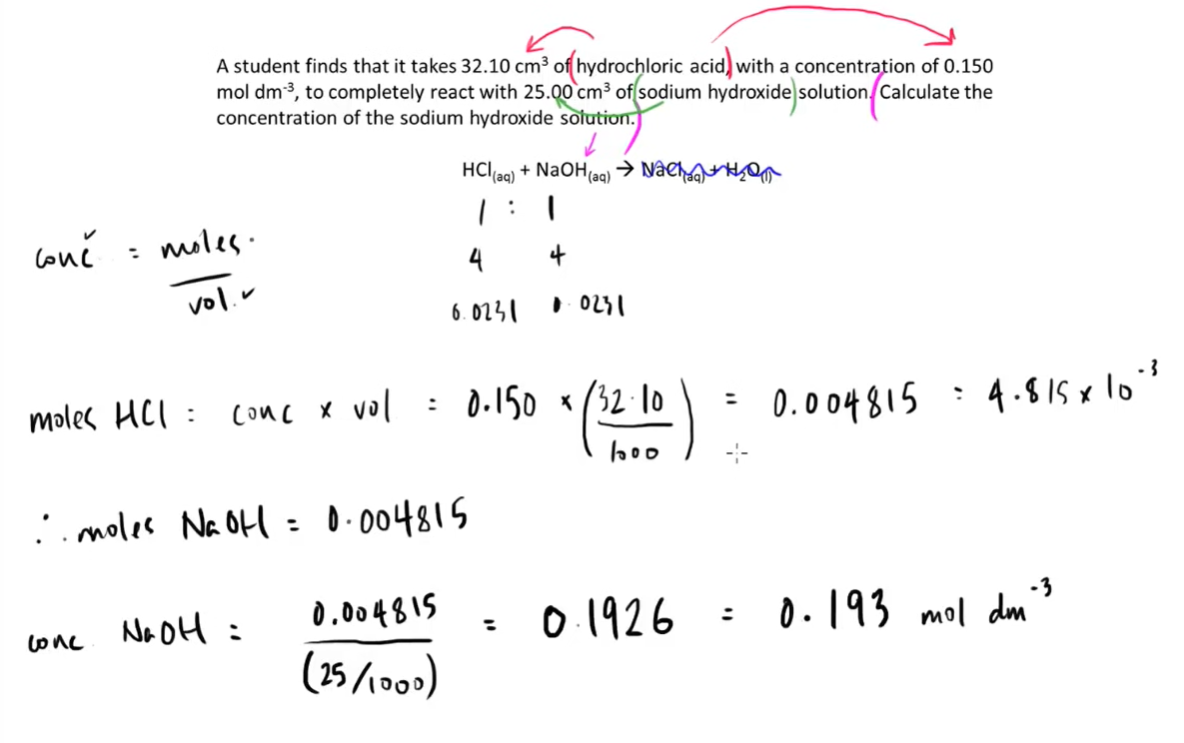
6
New cards

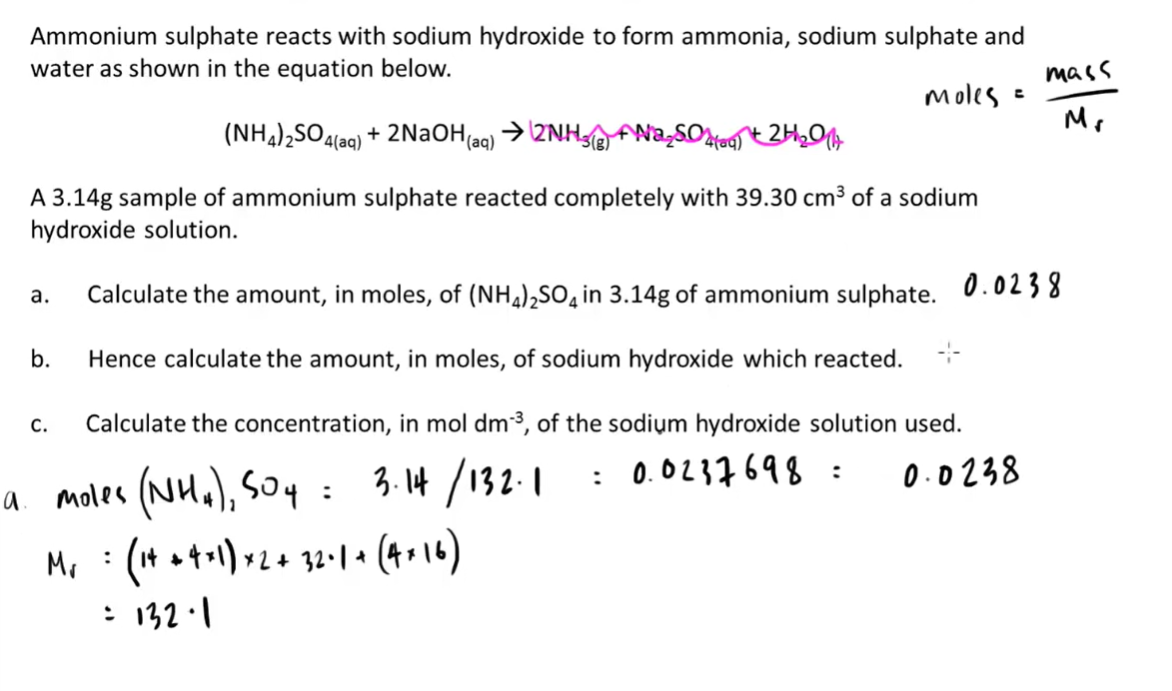
7
New cards

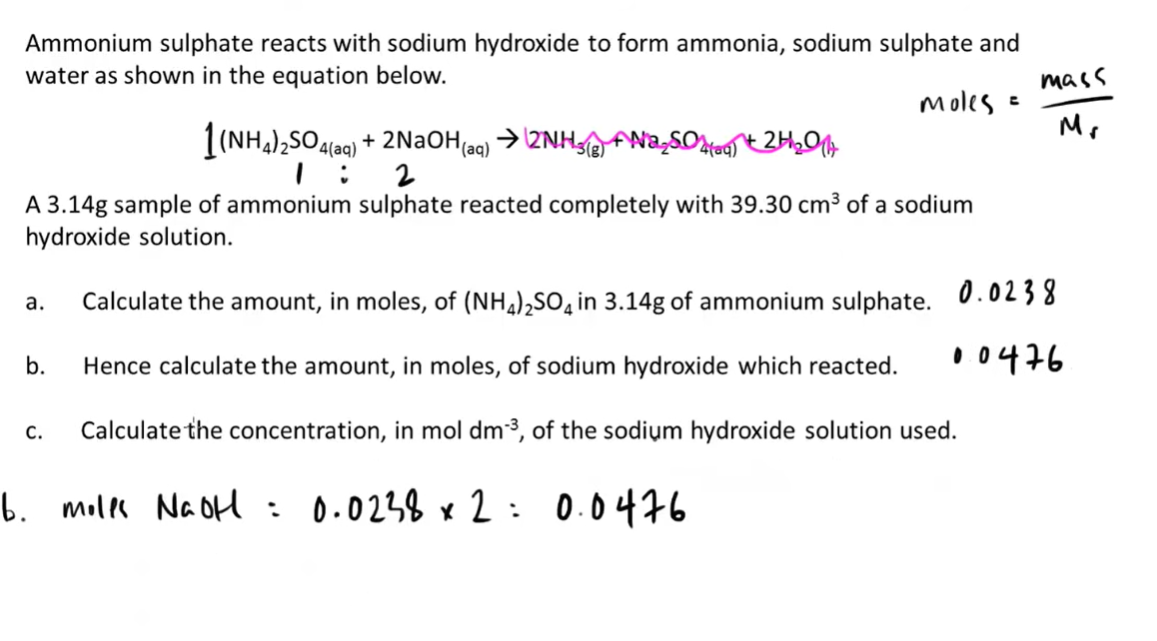
8
New cards
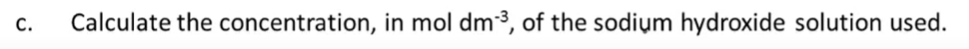
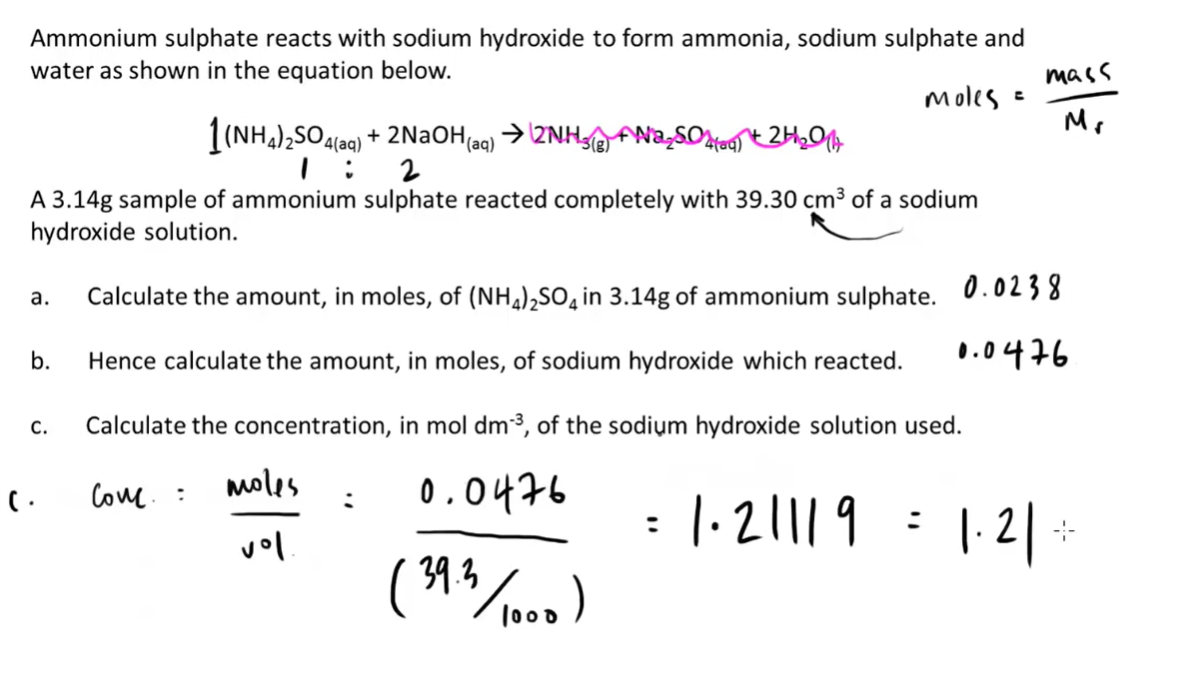
9
New cards