Science
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
State of Matter
The physical form matter takes — solid, liquid, gas, or plasma.
Solid
A state of matter with a definite shape and volume; particles are tightly packed and vibrate in place.

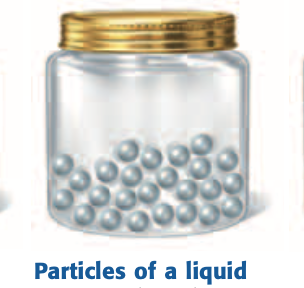
Liquid
A state of matter with a definite volume but no definite shape; particles slide past one another.
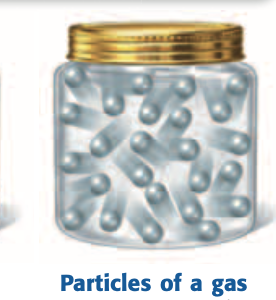
Gas
A state of matter with no definite shape or volume; particles move freely and spread far apart.
Plasma
A high-energy state of matter where gas particles become ionized (charged) and move extremely fast.
Ionized
When atoms lose or gain electrons and become charged particles called ions.
Particle Energy
The amount of energy particles have that determines how fast they move.
Melting
The change from a solid to a liquid when heat is added.
Freezing
The change from a liquid to a solid when heat is removed.
Evaporation
The process where a liquid slowly changes into a gas at its surface.
Boiling
The process where a liquid rapidly changes into a gas throughout the entire liquid.
Condensation
The process where a gas cools and changes into a liquid.
Sublimation
The change from a solid directly to a gas without becoming a liquid first.
Deposition
The change from a gas directly to a solid without becoming a liquid first.
Temperature
A measure of how fast the particles in a substance are moving.
Thermal Energy
The total energy of all the particles in a substance.
Heat
The transfer of thermal energy from something warmer to something cooler.
Change of State
When matter changes from one state (solid, liquid, gas, plasma) to another due to adding or removing heat.