Science in Action 9 Unit B 1.0-2.0
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
Toxic

Poisonous and infectious causing other toxic effects

Reactive Material

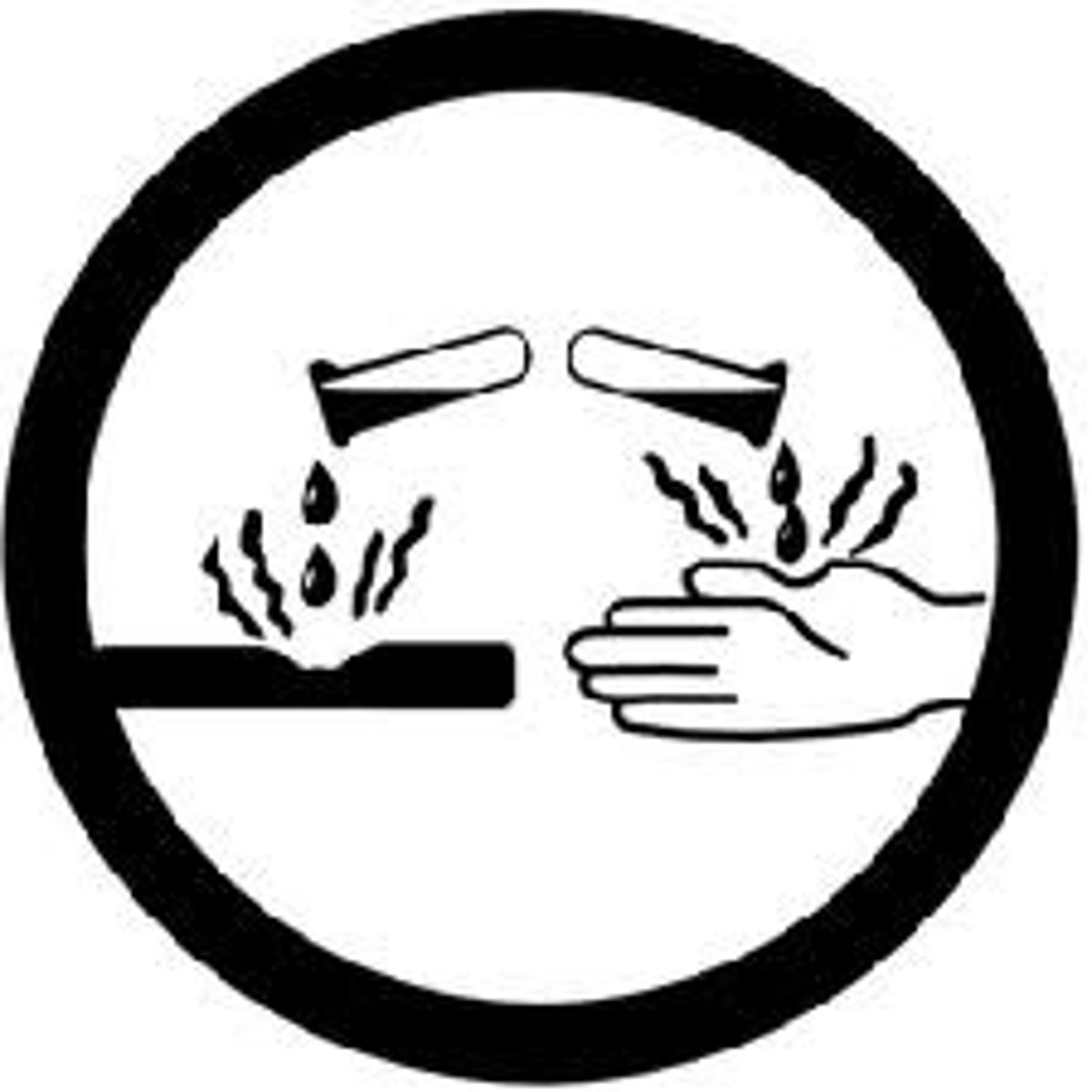
Corrosive Material
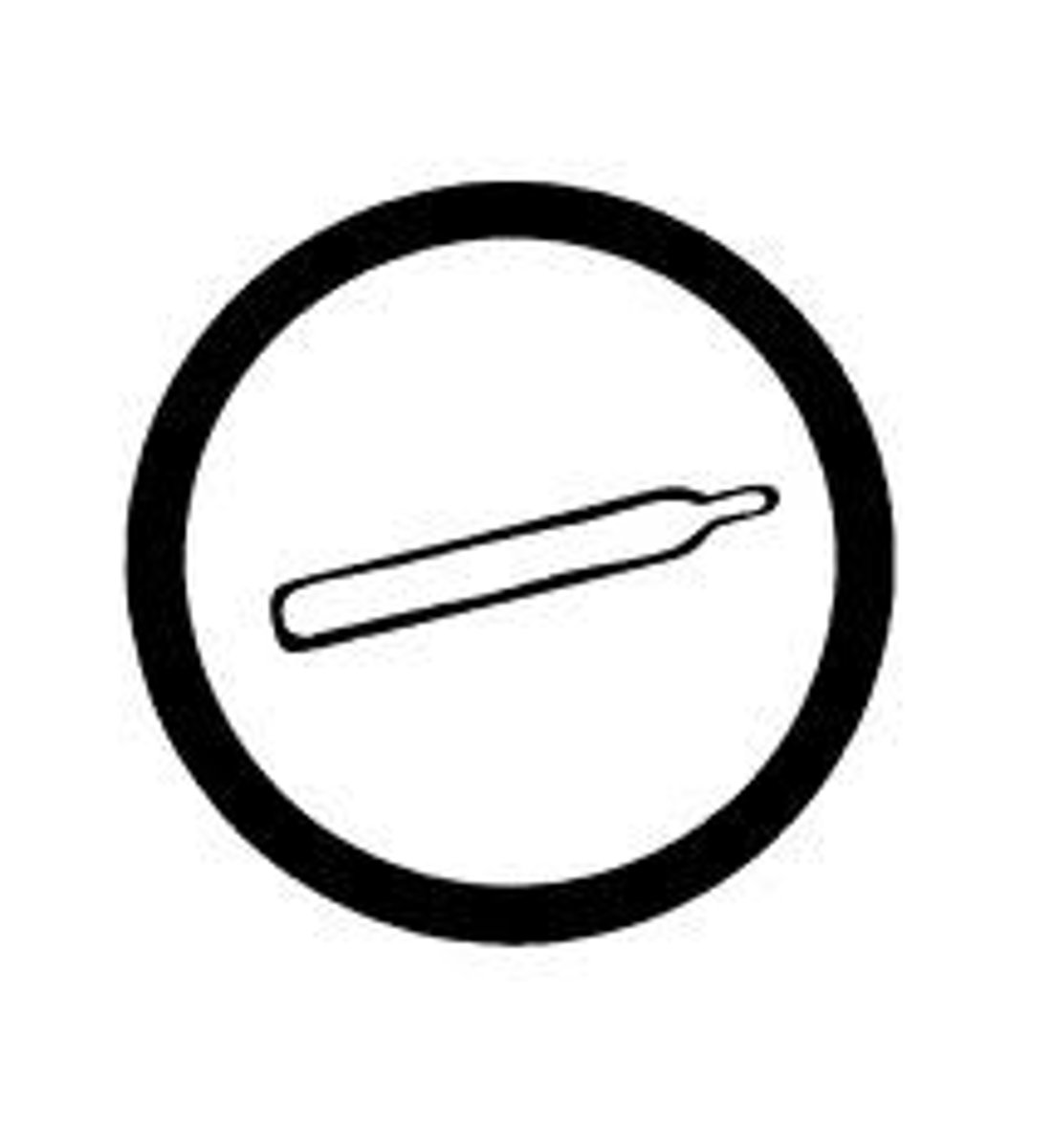
Compressed Gas
WHMIS
Workplace Hazardous Materials Information System
Gas to Liquid
Condensation
Solid to Gas
Sublimation
Liquid to Gas
Evaporation
Gas to Solid
Deposition
Liquid to Solid
Freezing
Solid to Liquid
Melting
Lustre
Shiny/glossy object
Malleability
Being able to shape an object without it breaking
Ductility
capable of being drawn out into wire or thread
Solubility
the amount of a substance that will dissolve in a given amount of another substance
Conductivity
how well can an object transmit and conduct electricity
Physical Change
occurs when a material changes state.
Chemical Change
when two or more substances react and create a new
substance.
Pure Substance
A substance that is only made up of one kind of matter.
Mixture
A substance that contains a combination of pure substances.
Element
Matter that cannot be broken down into any simpler substance
Compounds
Is a combination of two or more
elements in fixed proportions.
Colloids
a cloudy mixture, the particles are
so small that they cannot be filtered out easily.
Suspensions
a cloudy mixture in which tiny particles of one substance are held within another.
Solutions (Homogeneous Mixture)
Mixture that appear as one substance
Mechanical Mixture (Heterogeneous)
A Mixture where two or more substances are visible
Evidence of Chemical Change
Change in colour, change in odour, formation of a solid or gas, release or absorption of heat energy
How do you find how many protons or electrons are in each element.
Look at the atomic number, and that will tell you how many protons there are.
How to find how many neutrons are in an atom?
atomic mass-atomic number= number of neutrons
Warning
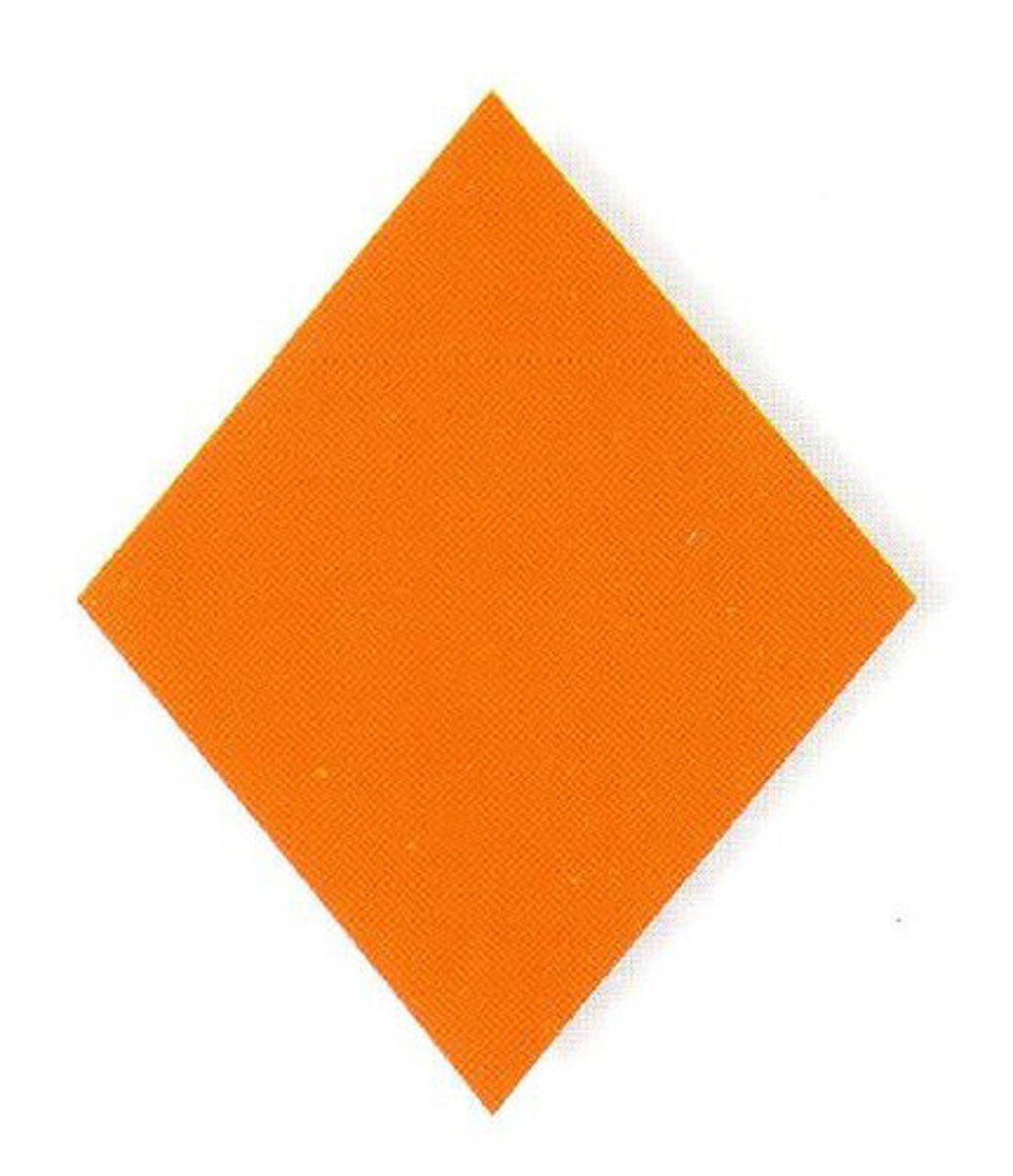
Caution

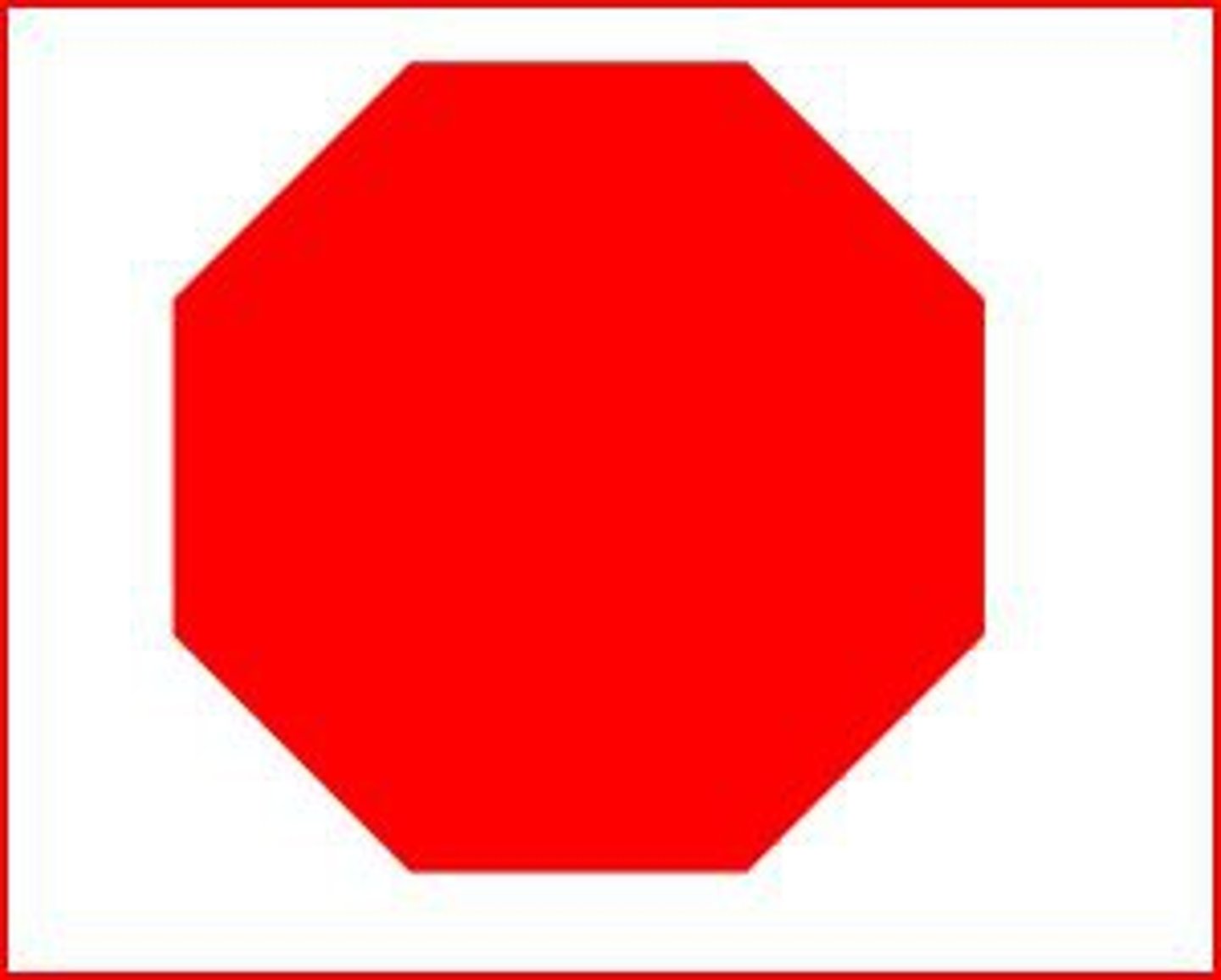
Danger
In the freeze drying method the 1st step is to.....
put it in a pressure chamber
Which symbol describes the danger of using an acid?

Matter is defined as anything that has mass and occupies...
Space
Density
Amount of madd in a given volume of a substance
Very reactive non-metals
Halogens
Have both properties of both metals and non-metals
Metalloids.
Very stable and non reactive elements
Noble gasses
Very reactive metals
Alkali Metals
Father of modern chemistry
Lavoisier
Aristotle
Matter was made up of earth, air, fire and water
J.J. Thompson model
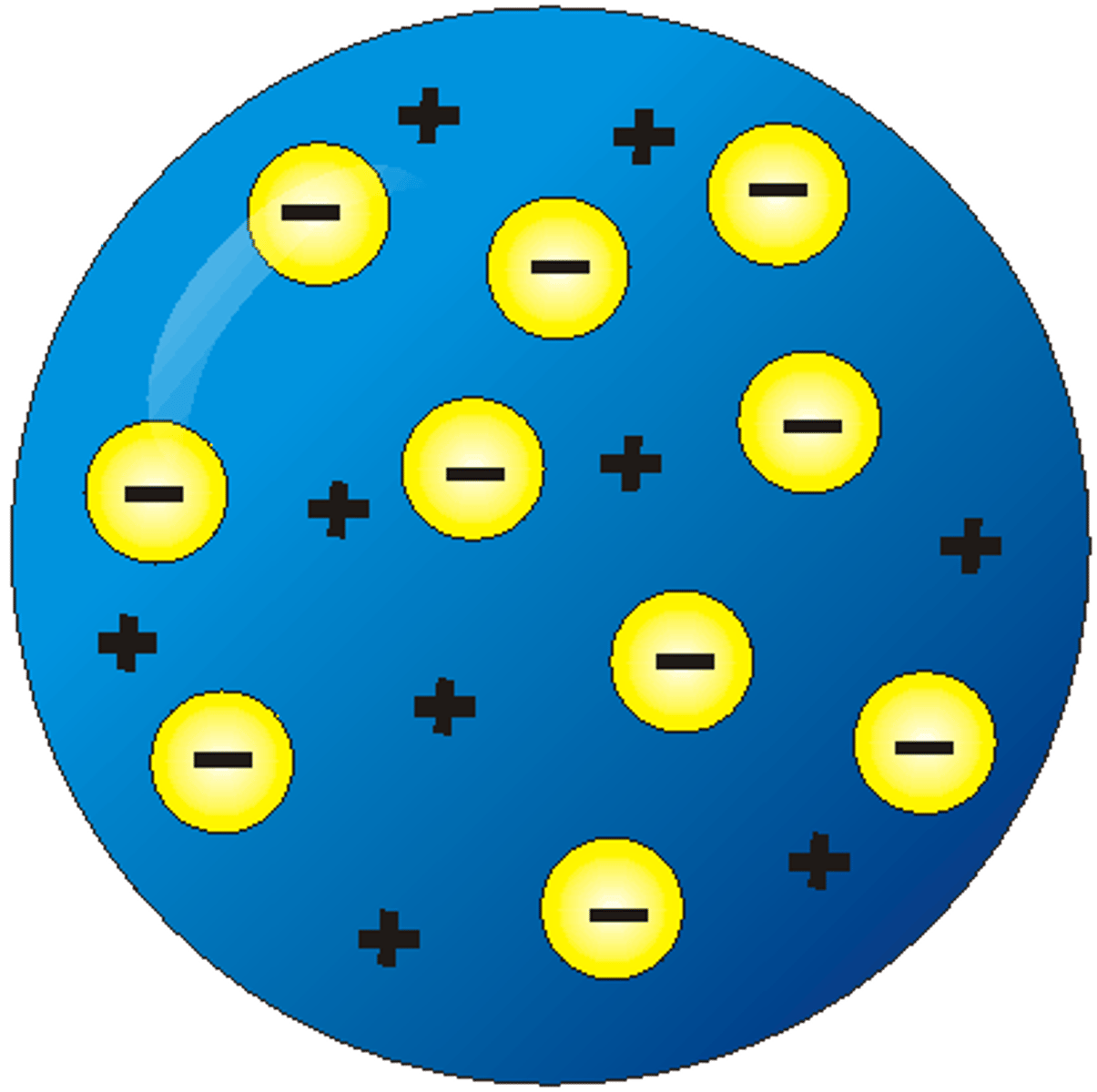
Hantaro Nagaoka's model
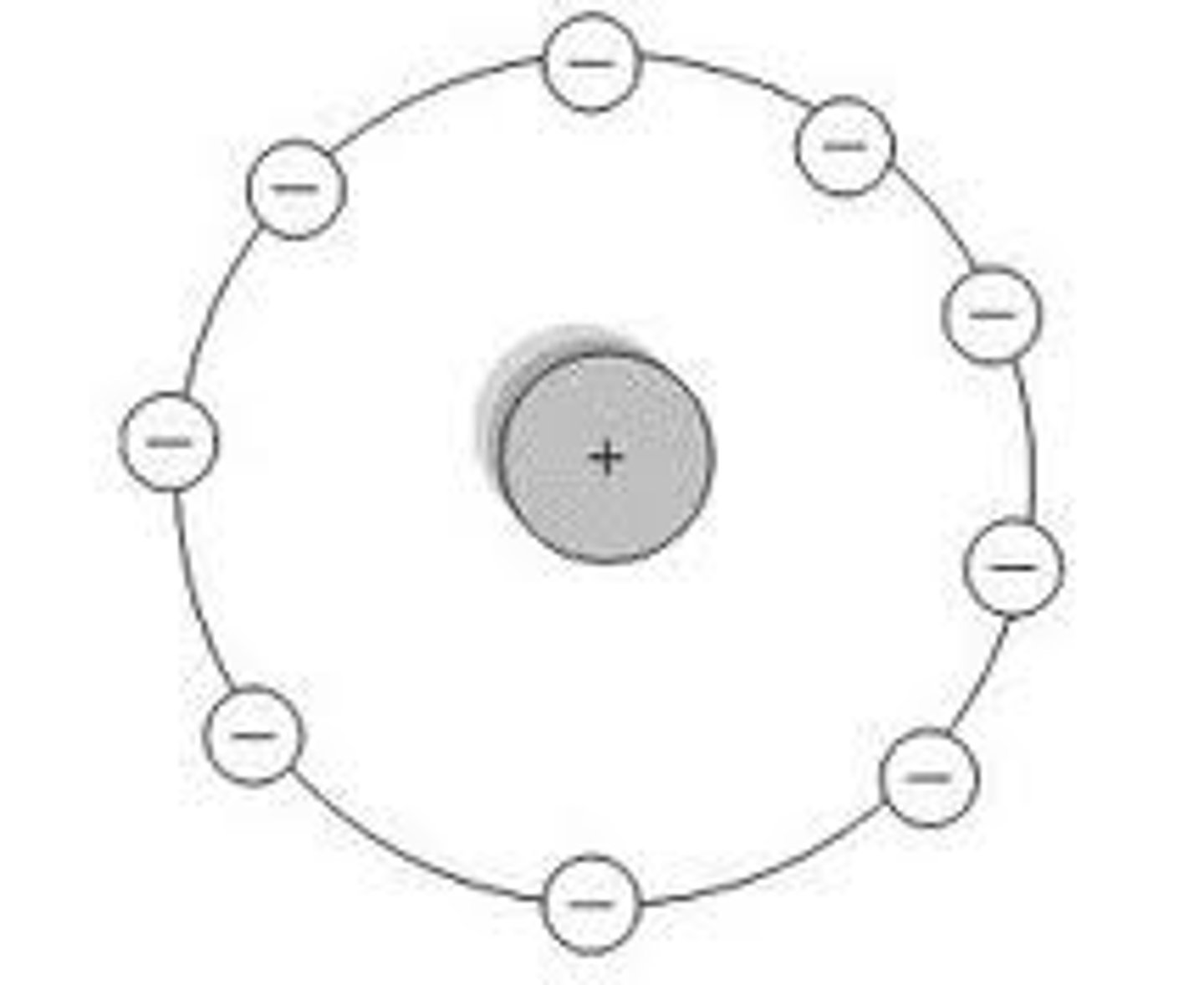
Who discovered Electrons?
J.J. Thompson
Bohr's model
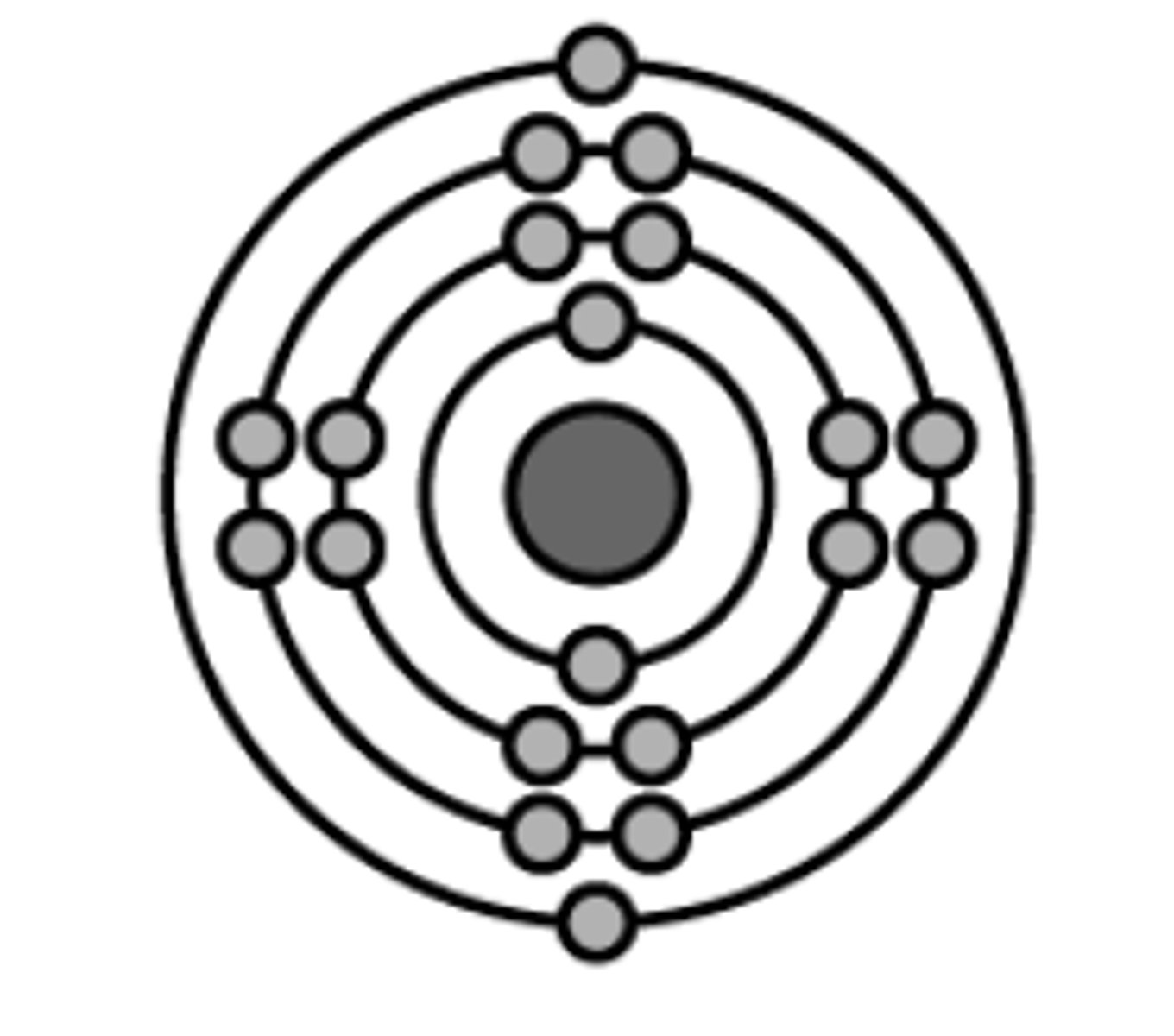
Dalton's model
Billiard ball model
What is the most numerous category of elements in the periodic table represent?
Metals
What does the atomic number of an atom represent?
Protons and electrons
Similar to a row of elements called a family
Groups
Families
Vertical Column of elements in the periodic table.
Peroids
Horizontal row of elements
Their reactivity is not as strong as the of the alkali metals
Alkaline-earth Metals