A Level Chem 1.09 Rate Equations
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
Solutions of two compounds, W and X, react together in the presence of a soluble catalyst, Y, as shown in the equation
2W + X → Z
When the concentrations of W, X and Y are all doubled, the rate of reaction increases by a factor of four.
Which is a possible rate equation for this reaction? (1)
A rate = k [W]2[X]
B rate = k [W]2[Y]
C rate = k [X] [Y]
D rate = k [X] [Z]
C (1)
If concentrations are doubled:
Order 1 → rate ×2
Order 2 → rate ×4
Order 3 → rate ×8
Since the rate increases by 4, the overall order must be 2.
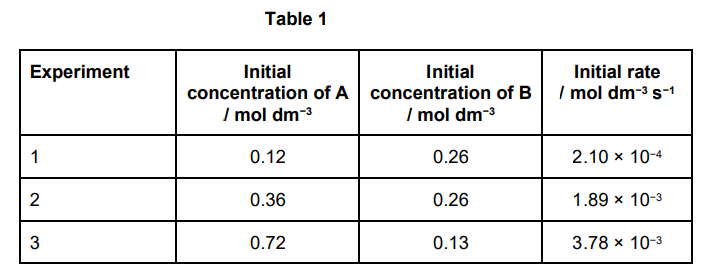
This question involves the use of kinetic data to deduce the order of a reaction and calculate a value for a rate constant. The data in Table 1 were obtained in a series of experiments on the rate of the reaction between compounds A and B at a constant temperature.
Show how these data can be used to deduce the rate expression for the reaction between A and B. (3)
Consider experiments 1 and 2:
[B constant] [A] increases × 3: rate increases by 32
therefore 2nd order with respect to A (1)
Consider experiments 2 and 3:
[A] increases × 2: rate should increase × 22
but only increases × 2
Therefore, halving [B] halves rate and so 1st order with respect to B (1) Rate equation: rate = k[A]2[B] (1)
This question is about rates of reaction.
Iodine and propanone react together in an acid-catalysed reaction
CH3COCH3(aq) + I2(aq) → CH3COCH2I(aq) + HI(aq)
A student completed a series of experiments to determine the order of reaction with respect to iodine.
Method
• Transfer 25 cm3 of 1.0 mol dm–3 propanone solution into a conical flask.
• Add 10 cm3 of 1.0 mol dm–3 HCl(aq)
• Add 25 cm3 of 5.0 × 10–3 mol dm–3
I2(aq) and start a timer.
• At intervals of 1 minute, remove a 1.0 cm3 sample of the mixture and add each sample to a separate beaker containing an excess of NaHCO3(aq)
• Titrate the contents of each beaker with a standard solution of sodium
thiosulfate and record the volume of sodium thiosulfate used.
(a) Suggest why the 1.0 cm3 portions of the reaction mixture are added to an excess of NaHCO3 solution. (2)
The sodium hydrogencarbonate solution neutralises the acid (catalyst) (1)
So stops the reaction (1)
This question is about rates of reaction.
Iodine and propanone react together in an acid-catalysed reaction
CH3COCH3(aq) + I2(aq) → CH3COCH2I(aq) + HI(aq)
A student completed a series of experiments to determine the order of reaction with respect to iodine.
Method
• Transfer 25 cm3 of 1.0 mol dm–3 propanone solution into a conical flask.
• Add 10 cm3 of 1.0 mol dm–3 HCl(aq)
• Add 25 cm3 of 5.0 × 10–3 mol dm–3
I2(aq) and start a timer.
• At intervals of 1 minute, remove a 1.0 cm3 sample of the mixture and add each sample to a separate beaker containing an excess of NaHCO3(aq)
• Titrate the contents of each beaker with a standard solution of sodium
thiosulfate and record the volume of sodium thiosulfate used.
Suggest why the order of this reaction with respect to propanone can be ignored in this experiment. (2)
The concentration/amount of propanone is much larger than/200 times larger than the concentration/amount of iodine (1)
Concentration of propanone is (almost) constant (1)
y axis-volume of sodium thiosulfate solution/cm3
x-axis-Time/mins
Explain how the graph shows that the reaction is zero-order with respect to iodine in the reaction between propanone and iodine (2)
The graph is a straight line / has a constant gradient (1)
So the rate of reaction does not change as the concentration (of iodine) changes (1)
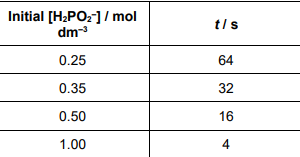
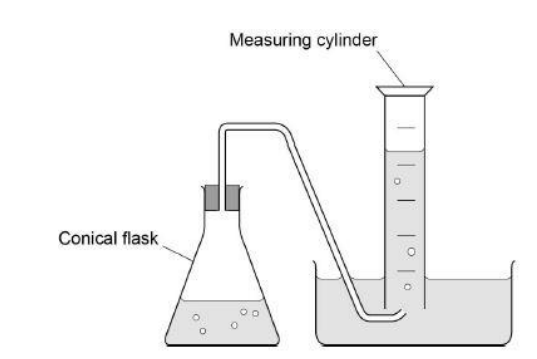
Another student reacted different initial concentrations of phosphinate ions with an excess of hydroxide ions.
The student measured the time (t) taken to collect 15 cm3 of hydrogen gas. Each experiment was carried out at the same temperature. The table shows the results.
State the relationship between the initial concentration of phosphinate and time (t). Deduce the order of the reaction with respect to phosphinate
[H2PO2 −]2 α 1/t (1)
Order =2 (1)
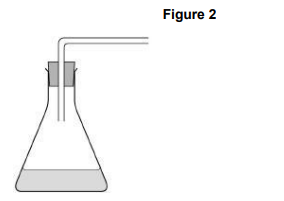
Complete the diagram in Figure 2 to show how the hydrogen gas could be collected and measured in the experiments (1)
(1)
The rate equation for a different reaction is rate = k [L][M]2
Deduce the overall effect on the rate of reaction when the concentrations of both L and M are halved. (1)
Multiplied by 1/8 OR falls by a factor of 8 (1)
Cisplatin, [Pt(NH3)2Cl2], is used as an anti-cancer drug.
After cisplatin enters a cell, one of the chloride ligands is replaced by a water molecule to form a complex ion, B.
Give the equation for this reaction.(2)
[Pt(NH3)2Cl2] + H2O → [Pt(NH3)2Cl(H2O)]+ + Cl− (2)
An experiment is done to investigate the rate of reaction.
[Pt(NH3)2Cl2] + H2O → [Pt(NH3)2Cl(H2O)]+ + Cl−
During the experiment the concentration of cisplatin is measured at one-minute intervals. Explain how graphical methods can be used to process the measured results, to confirm that the reaction is first order(3)
plot concentration (y-axis) against time (x-axis) and take tangents / (calculate the) gradients (to calculate rates) (1)
Plot rate/gradients against conc (1)
straight line through origin / directly proportional confirms first order (1)
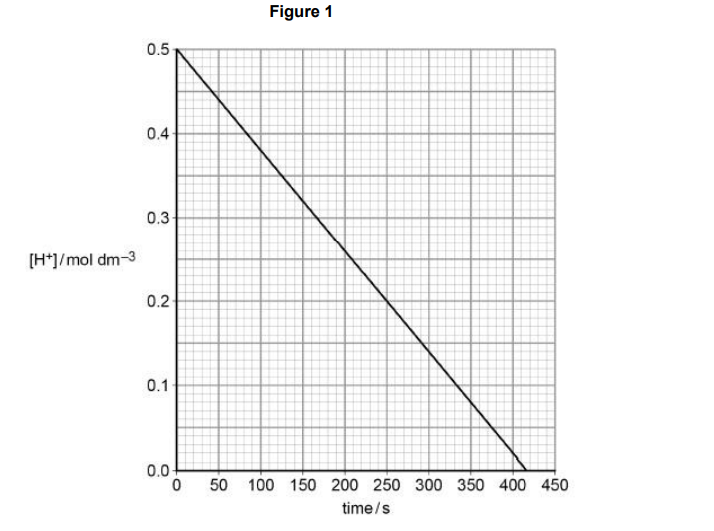
A graph of the results is shown in Figure 1.
Explain how the graph shows that the order with respect to H+ (aq) is zero (2)
constant gradient (1)
as [H+ ] changes/decreases (1)
A general equation for a reaction is shown.
A(aq) + B(aq) + C(aq) → D(aq) + E(aq)
In aqueous solution, A, B, C and D are all colourless but E is dark blue. A reagent (X) is available that reacts rapidly with E. This means that, if a small amount of X is included in the initial reaction mixture, it will react with any E produced until all of the X has been used up. Explain, giving brief experimental details, how you could use a series of experiments to determine the order of this reaction with respect to A. In each experiment you should obtain a measure of the initial rate of reaction. (6)
Level 3 5-6 marks All stages are covered and the explanation of each stage is correct and virtually complete.
Stage 1 Preparation
Measure (suitable/known volumes of) some reagents
Measure (known amount of) X into separate containers
Stage 2 Procedure
Start clock/timer at the point of mixing
Time recorded for appearance of blue colour
Use of same concentration of B and C
Same temperature/use water bath
Repeat with different concentrations of A
Stage 3 Use of Results
1/time taken is a measure of the rate
plot of 1/time against volumes/concentrations of A
![<p>Propanone reacts with bromine in alkaline conditions.</p><p>CH<sub>3</sub>COCH<sub>3</sub> + Br<sub>2</sub> + OH ⟶ CH<sub>3</sub>COCH<sub>2</sub>Br + Br<sup>–</sup> + H<sub>2</sub>O</p><p>The rate equation for this reaction is</p><p>Rate = k [CH<sub>3</sub>COCH<sub>3</sub>] [OH<sup>–</sup>]</p><p>Use evidence from the rate equation to explain why Step 1 is the rate determining step (1)</p>](https://knowt-user-attachments.s3.amazonaws.com/009b1e2c-43dd-47e3-9f05-64da58333c96.png)
Propanone reacts with bromine in alkaline conditions.
CH3COCH3 + Br2 + OH ⟶ CH3COCH2Br + Br– + H2O
The rate equation for this reaction is
Rate = k [CH3COCH3] [OH–]
Use evidence from the rate equation to explain why Step 1 is the rate determining step (1)
Step 1 includes CH3COCH3 and OH- and these are also in the rate equation
OR
Step 1 contains all the species in the rate equation (1)
The rate of reaction between calcium carbonate and hydrochloric acid is investigated using a continuous monitoring method.
Method
• Place a conical flask on a balance and add approximately 20 g of large marble chips.
• Add 50 cm3 of 0.4 mol dm–3 hydrochloric acid.
• Place a loose cotton wool plug in the neck of the flask.
• Zero the mass reading on the balance.
• Start a timer.
• Record the loss in mass (mt) every 30 seconds for 4 minutes. • Wait for the reaction to finish and record the total mass loss (mtotal).
• Plot a graph of (mtotal – mt) against time.
Suggest why a loose cotton wool plug is placed in the neck of the flask, instead of leaving the flask open or inserting a bung. (2)
to avoid (acid/solution/liquid) splashing/spraying/spitting (out) (1)
(The CO₂ gas bubbles form quickly and rise through the liquid. This bubbling can push tiny droplets of the acid out of the flask)
(Instead of inserting a bung) to allow gas/CO2 to escape (1) (for the mass loss)
The rate of reaction between calcium carbonate and hydrochloric acid is investigated using a continuous monitoring method.
Method
• Place a conical flask on a balance and add approximately 20 g of large marble chips.
• Add 50 cm3 of 0.4 mol dm–3 hydrochloric acid.
• Place a loose cotton wool plug in the neck of the flask.
• Zero the mass reading on the balance.
• Start a timer.
• Record the loss in mass (mt) every 30 seconds for 4 minutes. • Wait for the reaction to finish and record the total mass loss (mtotal).
• Plot a graph of (mtotal – mt) against time.
The mass of carbon dioxide produced in time t is equal to mt. The total mass of CO2 produced during the reaction is equal to mtotal. Explain why (mtotal – mt) is proportional to the concentration of hydrochloric acid remaining in the flask at time t. (2)
mtotal – mt is equal/proportional to (mass/amount of) CO2 still to be produced
CO2 still to be produced is proportional to (amount/concentration of) HCl still to react (1)
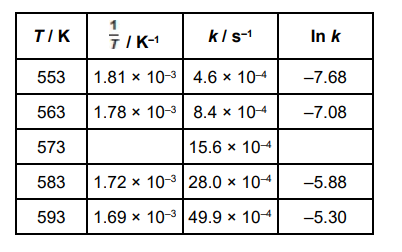
The thermal decomposition of but-3-en-1-ol is investigated at different temperatures (T). CH2=CHCH2CH2OH → CH2=CHCH3 + HCHO
The results from the investigation are used to calculate the rate constant, k, at each temperature. The table below shows some of the results.
The overall order of the reaction can be deduced from a piece of information in one of the column headings in the table. Identify this piece of information and deduce the overall order.
Piece of information __________________________________________
Overall order _______________________________________________(2)
Unit of k (is s-1) (1)
(order) 1/first (1)
k is independant of concentration for a first-order reaction
A and B react together in the presence of an acid catalyst.
A(aq) + 2 B(aq) → C(aq) + D(aq)
The rate equation for this reaction is rate = k[B]2[H+]
A suggested mechanism for the reaction is shown.
Step 1 B + H+ → BH+
Step 2 BH+ + B → B2H+
Step 3 B2H+ + A → C + D
Deduce the rate-determining step for this reaction. Give a reason for your answer. Rate-determining step ________________________________________
Reason ___________________________________________________(2)
Step 2 (1)
(By the end of step 2) 1 × H+ and 2 × B have been used (1)
For a chemical reaction the relationship between the rate constant, k, and
the temperature, T, is shown by the Arrhenius equation.
k=Ae^(-Ea/RT)
For the decomposition of gaseous ethanoic anhydride
the activation energy, Ea = 34.5 kJ mol–1
the Arrhenius constant, A = 1.00 × 1012 s–1
At temperature T1 the rate constant, k = 2.48 × 108 s–1
Calculate T1
The gas constant, R = 8.31 J K–1 mol–1 (3)
Ink=InA-Ea/RT (1)
T= Ea/(InA-Ink)*R (1)
T=500K (1)
An experiment was done to measure the time, t, taken for a solution of iodine to react completely when added to an excess of an acidified solution of butanone. Suggest an observation used to judge when all the iodine had reacted.(1)
Brown colour removed (1)
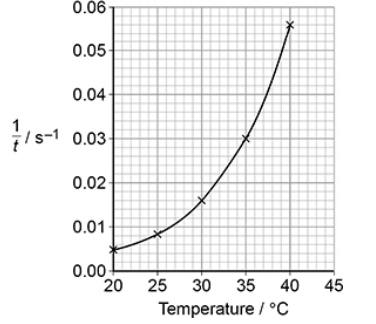
An experiment was done to measure the time, t, taken for a solution of iodine to react completely when added to an excess of an acidified solution of butanone.
The experiment was repeated at different temperatures. The graph below shows how 1/t varied with temperature for these experiments.
Describe and explain the shape of the graph above. (3)
As T increases rate (1/t) increases (1)
Exponentially (1)
Many more particles have E ≥ Ea (1)
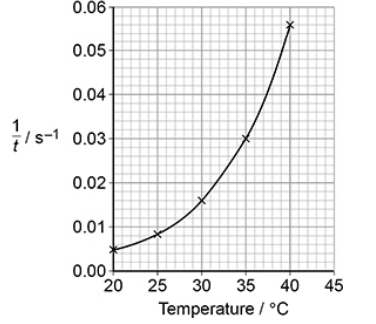
The experiment was repeated at different temperatures. The graph below shows how varied with temperature for these experiments.
Deduce the time taken for the reaction at 35 oC
Time =1/0.03=33s (1)
Hydrogen peroxide solution decomposes to form water and oxygen.
2 H2O2(aq) → 2 H2O(l) + O2(g)
The reaction is catalysed by manganese(IV) oxide.
A student determines the order of this reaction with respect to hydrogen
peroxide. The student uses a continuous monitoring method in the experiment.
The student places hydrogen peroxide solution in a conical flask with the catalyst
and uses a gas syringe to collect the oxygen formed. The student records the
volume of oxygen every 10 seconds for 100 seconds.
Explain why the reaction is fastest at the start. (2)
Higher/est concentration of / more H2O2 / particles / molecules / reactants (1)
More frequent successful collisions (1)