scientists
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
John Dalton (1805)
said atoms are indivisible (smallest particles) and had the solid sphere model. He also said all atoms of an element are identical (this is not true)
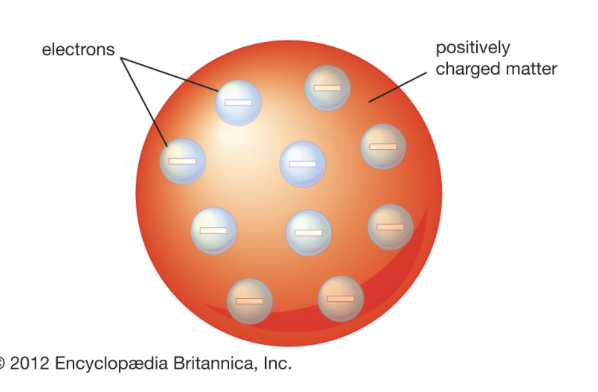
JJ Thompson (early 1900s)
performed the cathode ray experiment and came up with the “Plum Pudding model’ in 1904. The electrons of the atom are arranged orderly in a sphere of positive charge → look like plums in a plum pudding
Robert Millikan (1909)
performed the oil drop experiment where oil droplets would be suspended in an electric field to measure the charge of electrons. He discovered the negative charge of the electron that contributed to Rutherford and Bohr’s models later on
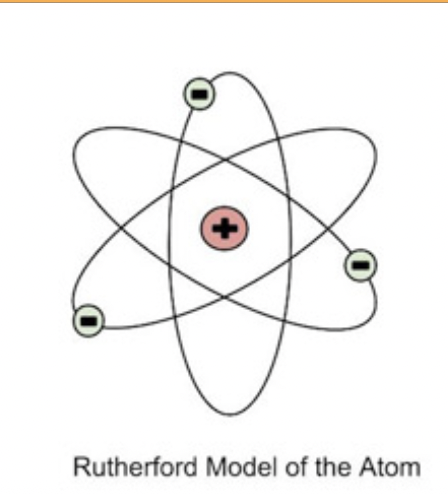
Robert Rutherford (1911)
aimed alpha particles (which have a positive charge) at a piece of gold foil. Most alpha particles went right through. This showed that the atoms were mostly empty space. However, some particles were deflected at large angles, leading Rutherford to conclude that atoms must have a small, dense, positively charged nucleus at their center.
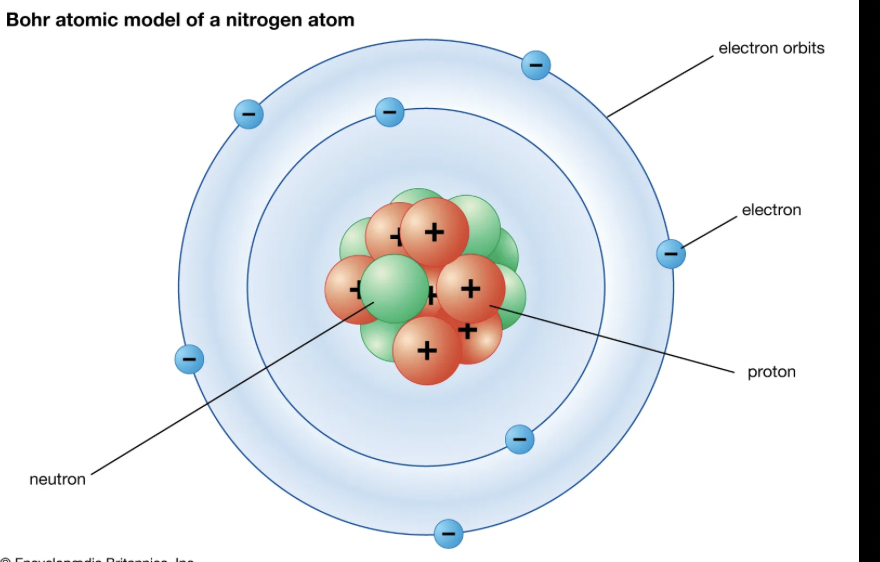
Niels Bohr (1913)
proposed that electrons exist in specific energy levels. When electrons "jump" between these levels, they emit or absorb light at specific wavelengths, explaining spectral lines and atomic stability (his experiment). Experimental data from hydrogen’s spectral lines helped Bohr propose that electrons occupy discrete energy levels. His new model kept the movement of the orbit to still rotate in a circular motion, however in a different patterns (no neutrons though)
Henry Moseley
used X-Ray spectroscopy to X-Ray elements, This allowed for him to view the relationship between atomic number and X-Ray frequency. His discovery led to the reordering of the periodic table from being based on the atomic mass to atomic number.
Marie Curie
discovered that radioactivity comes from atoms, revealing that certain elements emit radiation through the decay of their nuclei.
James Chadwick
discovered the neutrons present in the nucleus