chem semester 1 final
5.0(1)
Card Sorting
1/211
Last updated 9:20 PM on 12/10/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
212 Terms
1
New cards
specific heat
energy required to change an object's temperature by a certain amount
2
New cards
when heating equal amounts of oil and water, which substance will change temperature more? and why?
oil will change temperature more, the particles of the oil DO NOT resist the extra movement as much as water does
3
New cards
which substance has the highest specific heat?
water
4
New cards
substance A and B have equal mass. after hearing for 1 min, their temps are A = 50 C and B = 23 C. what is true?
substance a has a lower specific heat than b
5
New cards
substance a and b have equal mass. after heating for 1 min, their temps are A = 77 C and B = 105 C. what is true?
substance A has a higher specific heat than B
6
New cards
substance a and b have equal mass. after heating for 1 min their temps are A =77 C and B = 77 C. what is true?
they have equal specific heats
7
New cards
substance A and B have equal specific heat. after heating for 1 min, temps are A = 33 C and B = 77 C. what is true?
substance A has more mass than B
8
New cards
Liquids A and B have equal mass and specific heat. initial temps are A = 50 C and B = 0 C. after mixing A and B the final temp should be about ...
25 C
9
New cards
"The water has a temperature of 32 C after the cheeto is burned."
The observation is a _____ observation about a ____ property .
The observation is a _____ observation about a ____ property .
quantitative, physical
10
New cards
How many songs will you have listened to after 4 bathroom breaks?
Here are a few details about the trip.
Trip length = 90 miles
Money in pocket = $12.00
Time of trip = 75 minutes
Bathroom breaks = 1 break
Fuel used = 3 gallons of gasoline
Length of Playlist = 27 songs
Here are a few details about the trip.
Trip length = 90 miles
Money in pocket = $12.00
Time of trip = 75 minutes
Bathroom breaks = 1 break
Fuel used = 3 gallons of gasoline
Length of Playlist = 27 songs
27 songs per 1 bathroom break
11
New cards
1 meter = 3.3 feet
How many feet are in 10.5 meters?
How many feet are in 10.5 meters?
34.6
12
New cards
1 meter = 3.3 feet
12 inches = 1 foot
How many inches are in 91.5 meters?
12 inches = 1 foot
How many inches are in 91.5 meters?
3,623.4
13
New cards
0.18 grams of Cheeto = 1 Calorie
1 serving size of Cheeto = 227 grams
How many Calories are in 6.8 serving sizes of Cheeto?
1 serving size of Cheeto = 227 grams
How many Calories are in 6.8 serving sizes of Cheeto?
8,575.6
14
New cards
How many significant figures does the value 0.0101 mL have?
3
15
New cards
How many significant figures does the value 12.010 grams have?
5
16
New cards
Suppose you've measured one volume as 40.5 mL. You are going to measure one other volume, and then add them together. No matter what the other volume is, the sum cannot
go past the 10ths place
17
New cards
If 262 grams of water is to be heated from 18°C to 90°C to make a cup of tea, how much heat must be added? _____ J
The specific heat of water is 4.18 J/g °C
The specific heat of water is 4.18 J/g °C
78,852
18
New cards
When a block of aluminum loses 11,500 Joules of heat, its temperature changes from 66°C to 22°C. The specific heat of aluminum is 0.89 J / g °C.
What is the mass of the block? _________ g
What is the mass of the block? _________ g
294
19
New cards
A 5 kilogram block of wood has an original temperature of 6.2°C and a specific heat capacity of 2.40 J/g∙°C. What will be the final temperature of this wood if 68,870 J of heat energy are added? ______ °C
11.9
20
New cards
You take a 38.1 g sample of an unknown solid at 70.3°C and place it in 65.9 grams of water at 23.0°C. The equilibrium temperature of the mixture is 33.2°C. The specific heat of water is 4.18 J/g∙C
From this information, what is the specific heat of the solid? ______ J/g∙C
From this information, what is the specific heat of the solid? ______ J/g∙C
1.99
21
New cards
The neutrally charged parts of an atom
have a high mass and are located in the middle
22
New cards
The positively charged parts of an atom
have a high mass and are located in the middle
23
New cards
A particle with 9 protons, 7 neutrons, and 6 electrons would have a mass of ___ amu
16
24
New cards
A particle with 8 protons, 5 neutrons, and 1 electrons would have a charge of ____
7
25
New cards
Which observation provided evidence that the mass and positive charge within an atom was concentrated in a very small area?
Most (but not all) of the alpha particles went straight through the gold foil
26
New cards
Electrons that are closer to the nucleus have _____ potential energy than electrons that are further away from the nucleus.
less
27
New cards
When an electron in an excited state atom falls from high to low energy levels, the atom
emits radiation
28
New cards
When an atom absorbs radiation, its electrons
jump up from low to high energy levels
29
New cards
A greenhouse gas is a gas that ____ visible light and _____ infrared light. Examples include _____ and _____.
transmits, absorbs, carbon dioxide (CO2) and water vapor (H2O)
30
New cards
What is a true statement about the greenhouse effect?
It existed in the past and has increased and decreased over time, but is changing faster today than it has in the past
31
New cards
The majority of human-produced carbon dioxide is produced by
burning fossil fuels such as oil
32
New cards
Surfaces that are darker in color tend to have _______ albedos, and therefore reflect ______ light, than surfaces that are lighter in color.
lower, less
33
New cards
One reason that urban areas tend to get slightly hotter than rural areas is that urban areas have a _______ albedo, meaning they absorb _______ energy from sunlight.
lower, more
34
New cards
which two subatomic particles are always packed together in the center of the atom?
protons and neutrons
35
New cards
a positive calcium ion will _____ a neutral oxygen atom.
be attracted to
36
New cards
electrons in a higher energy level have _____ compared to electrons in lower energy levels
a weaker force of attraction to the nucleus and a lower ionization energy
37
New cards
what was the result if the gold foil experiment?
only a few of the alpha particles were deflected
38
New cards
in the gold foil experiment, some alpha particles got deflected by the nucleus, which indicated the nucleus was probably
positively charged
39
New cards
greenhouse gases absorb _____ radiation
infrared
40
New cards
ice reflects a lot of ____
sunlight
41
New cards
if the greenhouse effect didn't exist, earth would most likely
be too cold to live on
42
New cards
if an area has a high albedo that means that is
reflects a lot of visible light
43
New cards
after sitting outside on a hot afternoon, it will probably be cooler inside a white car than a red car because
the white car has a higher albedo
44
New cards
clouds during the day make it____, clouds at night make it ____.
cooler, warmer
45
New cards
how is polarization and ionization different from one another?
Polarization = why electrons move according to an external charged atom/object. Ionization = an electron entering/leaving the atom completely, gaining electrons give it a negative charge, while losing electrons give it a positive charge.
46
New cards
What are the 4 most important greenhouse gasses?
CO2 , H2O , CH4 , and N2O ; Carbon Dioxide, Water, Methane, and Nitrous Oxide
47
New cards
A positive feedback loop is when the effect ___ to the cause.
adds
48
New cards
A negative feedback loop is when the effect ___ the cause.
reduces
49
New cards
atomic number
the number of protons in the nucleus of the atom
50
New cards
mass number
the sum of the number of neutrons and protons in the nucleus
51
New cards
proton
charge of +1, mass of 1 amu, located in nucleus
52
New cards
electron
charge of -1, very small mass, located in orbitals
53
New cards
2 things that make Earth warmer:
sunlight getting absorbed, IR light getting absorbed and reemitted by gases in the atmosphere (greenhouse effect)
54
New cards
provided evidence about the mass of an electron
how much the cathode ray bent
55
New cards
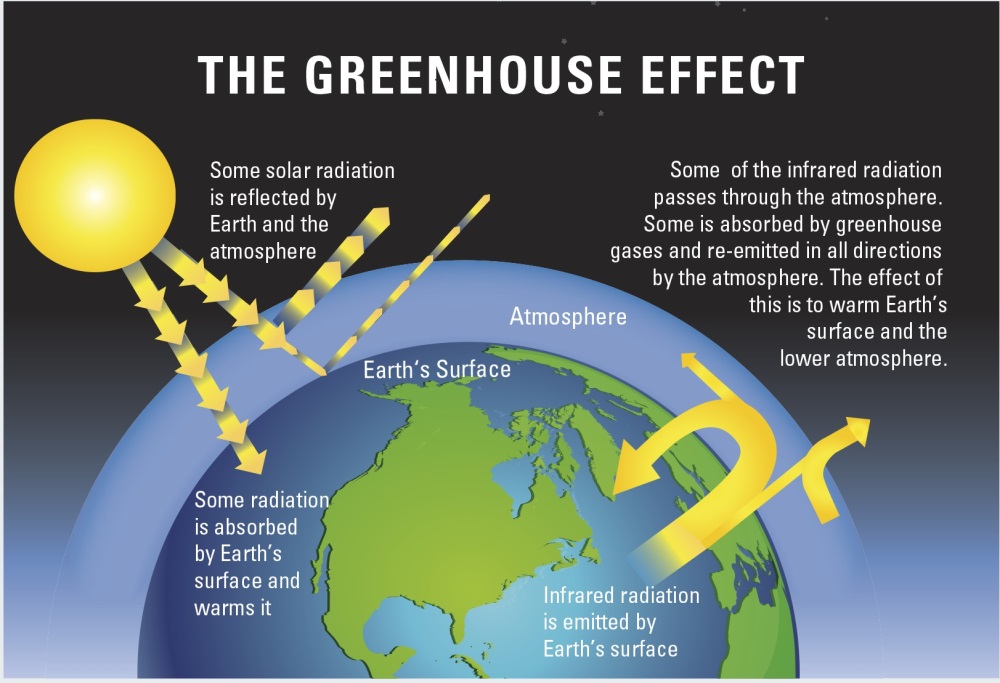
greenhouse effect
look at the picture :)
56
New cards
when sunlight gets to earth:
it gets reflected (mostly by clouds) OR it gets absorbed, and that energy will later be emitted from Earth's surface in the form of IR light
57
New cards
what is the mass of an atom if it has 20 protons, 22 electrons and 25 neutrons?
45 amu
58
New cards
what is the charge of an atom if it has 5 protons, 4 electrons and 3 neutrons?
+1
59
New cards
why don't all elements emit the same colors of light when they are energized
each element has different spacing between their energy levels
60
New cards
when an electron absorbs light energy it can
jump to a higher energy level or leave the atom completely
61
New cards
element a has a lot of blue lines in it's emission spectrum. element b only has 2 visible red lines. the energy levels ___
are probably farther apart in element a
62
New cards
why were the gold foil nuclei able to deflect the alpha particles?
because the electric field created by the nucleus is so concentrated
63
New cards
earth emits ____ radiation
infrared
64
New cards
when does atmospheric CO2 concentration peak each year?
May, because it is before plants have started to grow
65
New cards
in earth's geological past, what event triggered the start of ice ages?
earth tilting so the poles got less direct sunlight
66
New cards
during ice ages, oceans absorbed more CO2 as water got colder. This an a ___, and it made the temperature ____.
positive feedback loop, get even colder
67
New cards
What makes greenhouse gasses different from other gasses?
they absorb IR radiation and transmit visible light.
68
New cards
neutron
charge of 0, mass of 1 amu, located in nucleus
69
New cards
when sunlight gets to earth:
it gets reflected (mostly by clouds) OR it gets absorbed, and that energy will later be emitted from Earth's surface in the form of IR light
70
New cards
when IR is emitted from Earth:
it leaves through Earth's atmosphere and goes into space OR gets absorbed by molecules in the atmosphere and is converted to heat energy
71
New cards
2 things that make Earth cooler:
sunlight getting reflected and IR light leaving Earth
72
New cards
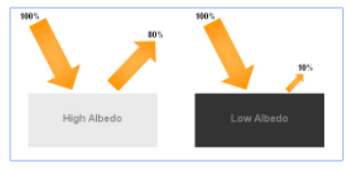
albedo
a quantity that measures how much of the light that hits a surface gets reflected
73
New cards
higher albedo
more light reflected, less light absorbed, stays COOLER
74
New cards
lower albedo
less light reflected, more light absorbed, gets HOTTER
75
New cards
positive feedback
when the effect adds to the cause
76
New cards
negative feedback
when the effect reduces the cause
77
New cards
positive or negative feedback?: as the planet warms, more ice melts, lowering the average albedo of Earth
positive feedback
78
New cards
positive or negative feedback?:
as the planet warms, the rate of evaporation from oceans increases, which increases the amount of water vapor in the atmosphere
as the planet warms, the rate of evaporation from oceans increases, which increases the amount of water vapor in the atmosphere
positive feedback
79
New cards
positive or negative feedback?:
as more CO2 dissolves in the ocean, algae populations will increase, so more photosynthesis will occur
as more CO2 dissolves in the ocean, algae populations will increase, so more photosynthesis will occur
negative feedback
80
New cards
positive or negative feedback?:
as droughts become more frequent and intense, more forests get destroyed, so there is less wet vegetation for water to evaporate from
as droughts become more frequent and intense, more forests get destroyed, so there is less wet vegetation for water to evaporate from
positive feedback
81
New cards
greenhouse gases transmit ____
visible light
82
New cards
the more charges there are, the _____ the attractive or repulsive force is
stronger
83
New cards
the number of energy levels in an atom depends on what ___ it is in
row
84
New cards
as you go down a column, atomic radius ___
increases
85
New cards
cation
positive ion
86
New cards
smallest common ions
Li+, Be+2, Na+, Mg+2
87
New cards
ionization energy
the energy needed to remove an electron from an atom
88
New cards
higher ionization energy means
that an electron in the atom is harder to remove
89
New cards
lower ionization energy means
that an electron in the atom is easy to remove
90
New cards
as you go down a column on the periodic table, ionization energy ________
decreases
91
New cards
why is it impossible to measure electronegativity for most of the noble gases?
they don't form bonds
92
New cards
more electronegative means that the atom
really wants electrons
93
New cards
as you go down a column on the periodic table, electronegativity ________
decreases
94
New cards
as you go across a row on the periodic table, electronegativity __________
increases
95
New cards
the lower the element's ____, the greater its reactivity
ionization energy
96
New cards
why bigger atoms have lower ionization energy and high reactivity
The bigger an atom is, the farther its valence electrons are from the positively charged nucleus, so the weaker the force of attraction is between its valence electrons and the nucleus, so the easier it is to remove an electron from the atom. Therefore, the bigger a metal atom is, the lower its ionization energy is, and the higher its reactivity is.
97
New cards
for the metals, reactivity ____ as you go down a column of the periodic table
increases
98
New cards
the greater the element's ____, the greater its reactivity
electronegativity
99
New cards
why is the bigger a nonmetal atom is, the lower its electronegativity is, and the lower its reactivity is
the bigger an atom is, the farther its valence electrons are from the positively charged nucleus, so the weaker the force of attraction is between its valence electrons and the nucleus, so the weaker the atom pulls on an attracts other electrons
100
New cards
for the nonmetals, reactivity ____ as you go down a column of the periodic table
decreases
Explore top notes
Biology 120 Notes (Part 9) Phospholipids, Plasma Membrane, Diffusion, and Osmosis
Updated 1266d ago0.0(0)
Biology 120 Notes (Part 9) Phospholipids, Plasma Membrane, Diffusion, and Osmosis
Updated 1266d ago0.0(0)