Molecular Geometry Quiz
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
What is a linear molecule and its degree?
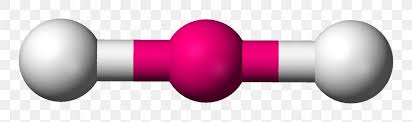
180 degrees
What is a Trigonal Planar and its degree?
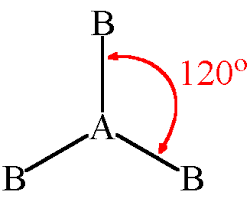
120 degrees
What is a Tetrahedral and its degree?
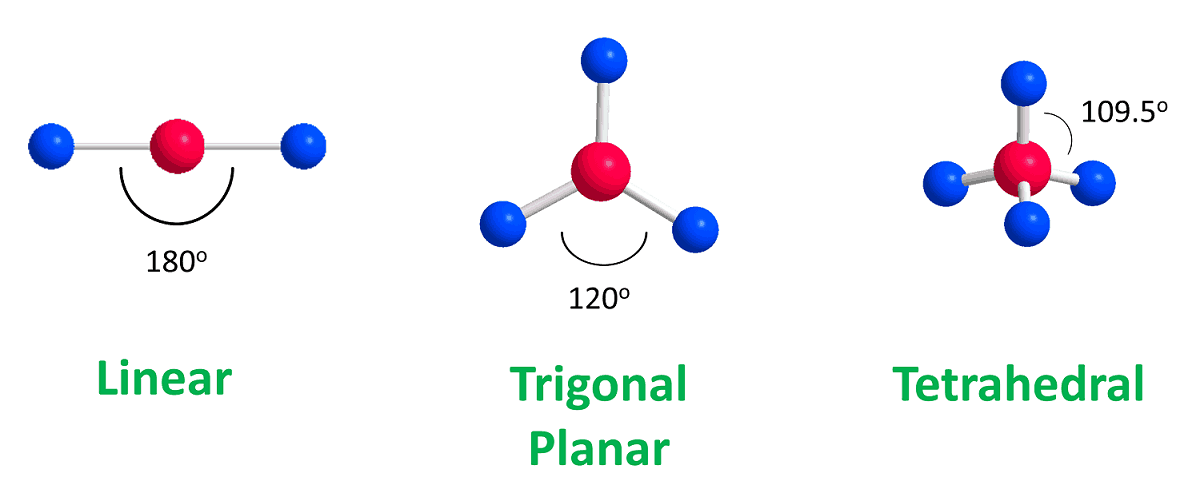
109.5 degrees
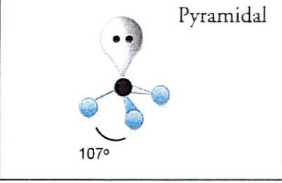
What is a Pyramidal?
107 degrees
What is bent?
104.5 degrees
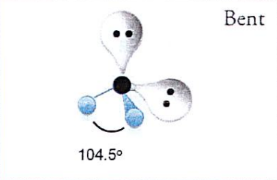
What is the second form of bent?
120 degrees

In determining the shape, what should you check?
You should look for bonds or lone pairs on central atom. For example, 3 bonds and one lone pair on the central tom is Pyramidal.
A molecule will be nonpolar if the molecular shape around the central atom has no lone pairs, or if it does, it’s either square planar or linear.
And if all atoms around the central atom are the same.
If not true, then it’s polar.
How to determine if a moelcule is non polar or polar.