Plasma Membranes
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Phospholipid Bilayer
Hydrophilic phosphate heads form outer + inner surfaces.
Hydrophobic fatty acid tails form the core.
Amphipathic nature → forms a selective barrier.
Cell membranes exist in aqueous environments (cytosol and extracellular fluid).
Model for Membranes
Fluid mosaic model:
Phospholipids free to move → membrane flexibility.
Proteins embedded vary in shape, size, position → like a “mosaic”.
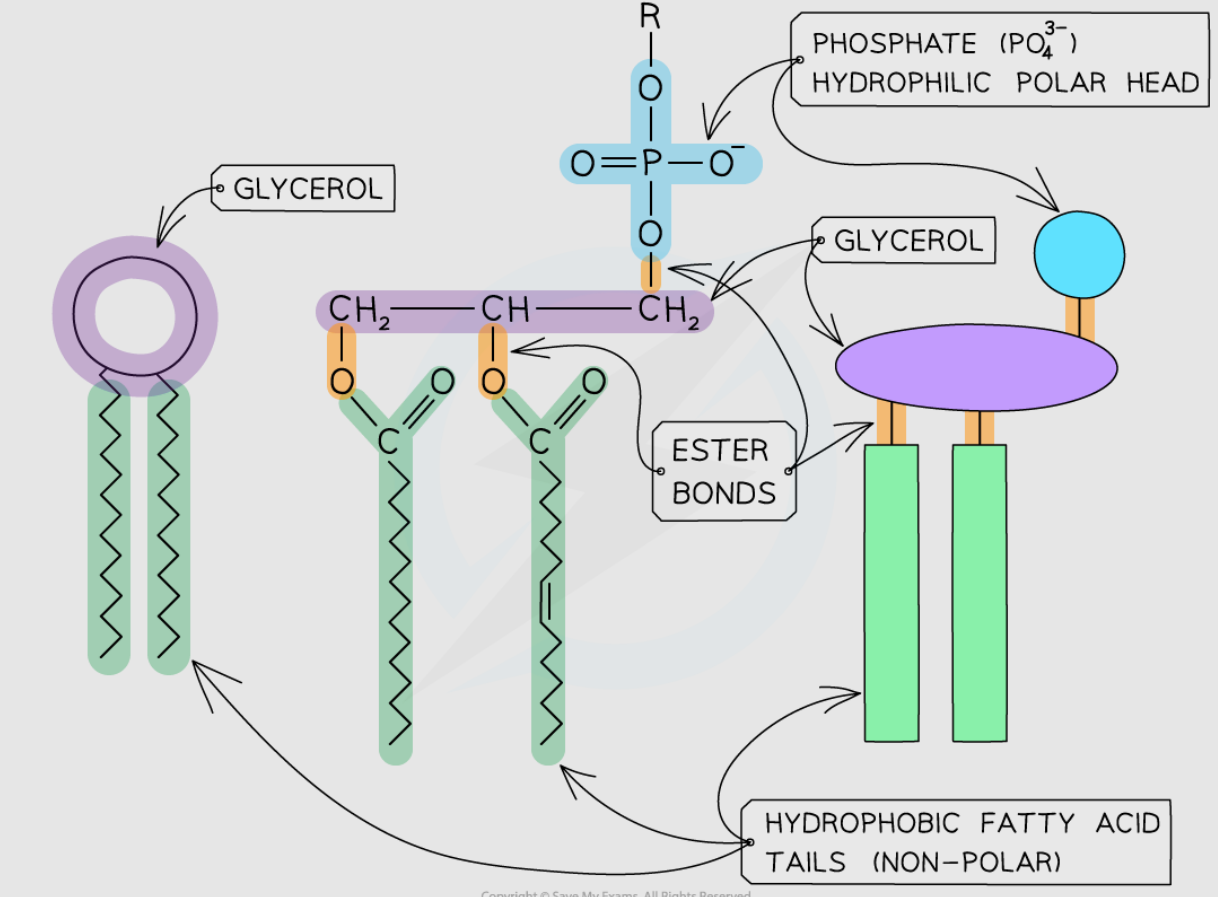
Phospholipid
Core structure of bilayer.
Movement of phospholipids → drives movement of other components.
Hydrophobic barrier prevents passage of water-soluble substances.
Intrinsic Protein
Two main types:
Intrinsic proteins (integral):
Transmembrane, embedded through bilayer.
Contain hydrophobic R groups that interact with core.
Channel Protein
→ provide hydrophilic channel for passive diffusion of polar molecules/ions (down conc. gradient).
Held together by the interactions of the hydrophobic core & R groups.
Carrier Protein
→ used for passive AND active transport (against gradient, shape change).
Also helps with facilitated diffusion
Extrinsic Protein
Present on one side of bilayer. (peripheral)
Hydrophilic R groups interact with phospholipid heads or intrinsic proteins.
Can be present in either layer or move between.
Usually involved in reactions and have enzymes attached to them.
Used in cell signalling or recognition.
Glycoproteins
Intrinsic proteins with carbohydrate chains attached.
Roles:
Cell adhesion.
Receptors for chemical signals (hormones, neurotransmitters, antibodies).
Example responses:
Neurotransmitter binding → triggers impulse.
Insulin/glucagon binding → regulates blood glucose.
Drugs (β-blockers) can bind to receptors.
Function: cell signalling/ communication/ recognition.
Glycolipids
Lipids with carbohydrate chains attached.
Roles:
Cell markers / antigens.
Identified by immune system as self / non-self.
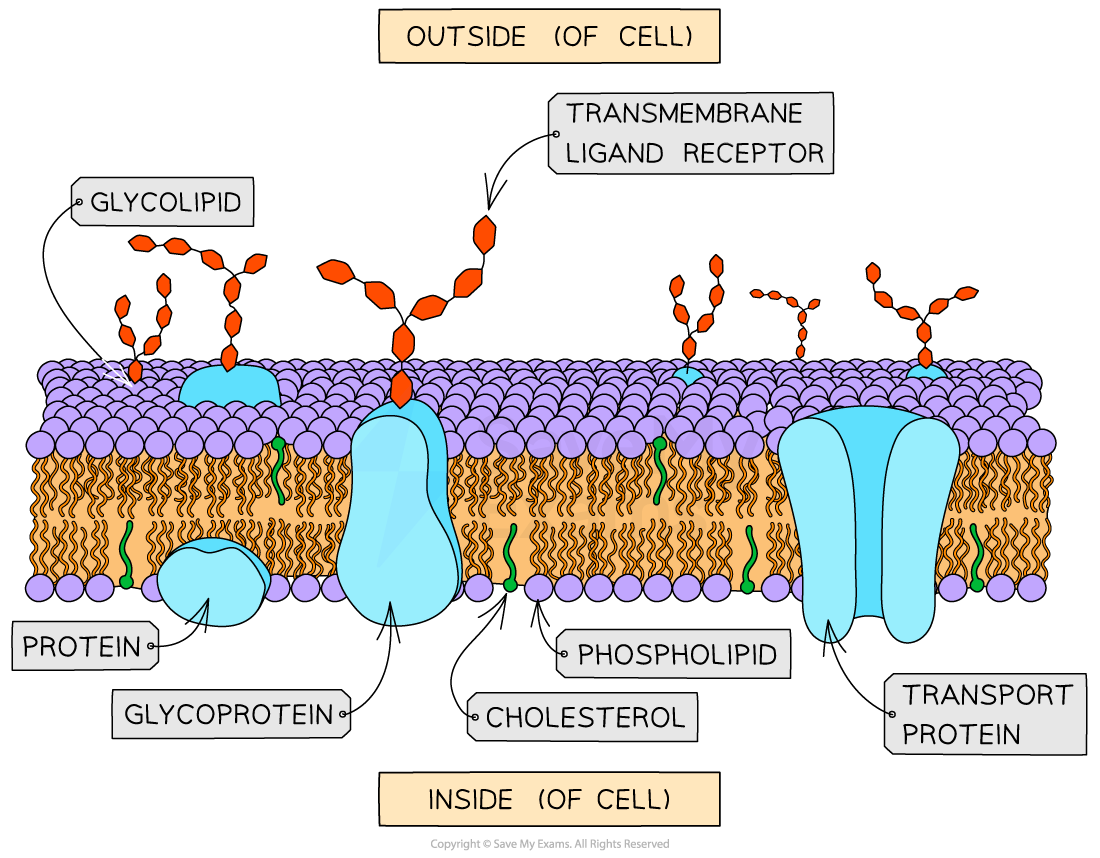
Cholesterol
Lipid with hydrophilic end + hydrophobic end.
Sits between phospholipids:
Hydrophilic end interacts with heads.
Hydrophobic end interacts with tails.
Roles:
Adds stability, prevents membranes becoming too rigid or bursting.
Reduces fluidity at high temperatures.
Prevent leakage of water & dissolved ions from cell.
Prevents phospholipids crystallising → maintains fluidity.
Functions of Plasma Membranes
At cell surface:
Separate cell contents from environment.
Selectively permeable barrier.
Allow cell communication/signalling.
Allow cell recognition.
Site of some chemical reactions.
Within cells:
Compartmentalisation of organelles (separate different areas within the cells from each other)
Form vesicles.
Control entry/exit of substances into organelles.
Provide reaction surfaces (e.g. inner mitochondrial membrane, thylakoid membranes).
Sites of Chemical Reactions
Many enzymes are bound to membranes.
Correct positioning is vital for reactions:
Mitochondria → cristae contain electron carriers + ATP synthase (respiration).
Chloroplasts → thylakoid membranes contain photosynthetic enzymes.
Factors affecting structure:
Temperature
Solvents
How does temperature affect?
Phospholipids: constantly moving.
Increased temperature → more kinetic energy → phospholipids move more → membrane becomes:
More fluid.
More permeable.
If temperature continues rising → phospholipid bilayer structure breaks down completely.
Result:
Easier for particles to cross membrane.
Carrier & channel proteins denature at high temps → membrane transport disrupted → increased permeability.
⚠ Study tip: Proteins are denatured by heat, but phospholipid membranes are described as disrupted or destroyed, not denatured.
How does solvent affect?
Water (polar solvent): essential for phospholipid bilayer formation.
Hydrophilic phosphate heads interact with water.
Hydrophobic tails cluster away from water → bilayer integrity maintained.
Organic solvents (less polar or non-polar, e.g. alcohol, benzene):
Dissolve membranes → disrupt cell membranes.
Alcohols used in antiseptic wipes: dissolve membranes of bacteria → kill them → reduced infection risk.
Low conc. alcohols (e.g. alcoholic drinks):
Don’t fully dissolve membranes but still disrupt.
Non-polar molecules enter bilayer, creating gaps → membrane becomes more permeable.
Physiological effect: Neurone membranes disrupted → nerve impulse transmission affected → altered behaviour after alcohol.
Practical investigation – beetroot membrane permeability
Why beetroot? Contains betalain pigment (red). Released if cell membranes disrupted → solution colour changes.
Method:
Equal beetroot discs prepared, washed in running water.
Placed in 100 ml distilled water at different temps (water bath, 10°C intervals).
At each temp, 5 samples of surrounding solution taken.
Absorbance of pigment measured by colorimeter with blue filter.
Experiment repeated ×3, mean absorbance calculated.
Results:
Graph shows increasing absorbance with rising temperature.
Point where curve rises steeply = membrane disruption threshold.