Some Questions
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
A sample of glucose (C₆H₁₂O₆) has a mass of 9.00 g. How many oxygen atoms are in the sample?
(A) 1.81 × 10²²
(B) 3.01 × 10²²
(C) 1.81 × 10²³
(D) 6.02 × 10²²
Solution:
Molar mass C₆H₁₂O₆ = 180.16 g/mol
moles = 9.00 / 180.16 ≈ 0.0500 mol
Each mole has 6 moles O → 0.0500 × 6 = 0.300 mol O
Atoms of O = 0.300 × 6.022×10²³ = 1.81×10²³
Answer: (C) 1.81 × 10²³
The mass spectrum of element X shows two peaks:
69 amu (60.1% abundance)
71 amu (39.9% abundance)
What is the average atomic mass of X?
(A) 69.2 amu
(B) 70.0 amum
(C) 69.8 amu
(D) 69.5 amu
Solution:
(69 × 0.601) + (71 × 0.399) = 41.469 + 28.329 = 69.8 amu
Answer: (C) 69.8 amu (Gallium)
What is the percent by mass of oxygen in potassium chlorate, KClO₃?
(A) 39.2%
(B) 47.5%
(C) 51.6%
(D) 62.3%
Solution:
Molar mass KClO₃ = 39.1 + 35.45 + 48.00 = 122.55 g/mol
Mass of O = 48.00 g
% O = (48.00 / 122.55) × 100 ≈ 39.17%
Answer: (A) 39.2%
An ion has 18 electrons, 18 neutrons, and a charge of –1. What is its mass number?
(A) 35
(B) 36
(C) 37
(D) 38
Solution:
Charge = –1 → gained 1 electron → neutral atom had 17 e⁻ → atomic number Z = 17 (chlorine)
Protons = 17
Neutrons = 18
Mass number A = 17 + 18 = 35
Answer: (A) 35 → chloride-35
Which of the following represents the ground-state electron configuration of Fe³⁺?
(A) [Ar] 4s² 3d³
(B) [Ar] 4s⁰ 3d⁵
(C) [Ar] 4s¹ 3d⁴
(D) [Ar] 4s² 3d⁶
Solution:
Fe (Z=26): [Ar] 4s² 3d⁶
Fe³⁺ loses 3 electrons: first 4s², then one from 3d → [Ar] 3d⁵ → same as (B)
Answer: (B) [Ar] 4s⁰ 3d⁵
Combustion of a hydrocarbon produces 4.40 g CO₂ and 2.70 g H₂O. What is the empirical formula?
(A) CH
(B) CH₂
(C) CH₃
(D) C₂H₅
Solution:
mass C = 4.40 × (12.01/44.01) ≈ 1.20 g
mass H = 2.70 × (2.016/18.02) ≈ 0.302 g
moles C = 1.20 / 12.01 ≈ 0.100
moles H = 0.302 / 1.008 ≈ 0.299
ratio H:C = 0.299 / 0.100 ≈ 3 → CH₃
Answer: (C) CH₃
A PES spectrum shows three peaks with relative areas 2:2:6. The highest binding energy peak corresponds to n=1. What element is this?
(A) Li
(B) C
(C) O
(D) Ne
Solution:
Peak areas = # electrons per subshell
n=1 (highest BE) = 2 e⁻ → 1s²
Next = 2 e⁻ → 2s²
Last = 6 e⁻ → 2p⁶
Total e⁻ = 2+2+6 = 10 → neon (Ne)
Answer: (D) Ne
Which sequence correctly orders the elements by increasing first ionization energy?
(A) Na < Mg < Al < Si
(B) Na < Al < Mg < Si
(C) Si < Al < Mg < Na
(D) Al < Na < Si < Mg
Solution:
IE increases across period, but Al < Mg due to s→p drop
Order: Na < Al < Mg < Si
Answer: (B) Na < Al < Mg < Si
Which compound has the highest melting point?
(A) NaCl
(B) MgO
(C) KBr
(D) CsI
Solution:
Melting point ↑ with ↑ ionic bond strength → depends on charge product (q₁q₂)
NaCl: (+1)(–1) = 1
MgO: (+2)(–2) = 4 → strongest lattice
Size also matters — Mg²⁺ and O²⁻ are small → even stronger
Answer: (B) MgO
A photon has wavelength 486 nm. To which region of the spectrum does it belong?
(A) Ultraviolet
(B) Visible
(C) Infrared
(D) Microwave
Solution:
Visible light = ~400–700 nm
486 nm → blue-green light → visible
Answer: (B) Visible
A 2.50 g sample of an unknown hydrocarbon is completely burned in excess oxygen. The products are passed through two traps:
Trap 1 absorbs water and gains 3.60 g.
Trap 2 absorbs carbon dioxide and gains 8.80 g.
(a) Calculate the mass of carbon in the original sample.
(b) Calculate the mass of hydrogen in the original sample.
(c) Determine the empirical formula of the hydrocarbon.
(d) If the molar mass of the compound is 56.1 g/mol, what is its molecular formula?
(e) Is this compound likely ionic or covalent? Justify your answer based on bonding and physical properties.
(a) Calculate the mass of carbon in the original sample.
mass C = 8.80 g CO₂ × (12.01 g C / 44.01 g CO₂) = 2.40 g C
Answer: 2.40 g
(b) Calculate the mass of hydrogen in the original sample.
mass H = 3.60 g H₂O × (2.016 g H / 18.02 g H₂O) = 0.403 g H
Answer: 0.403 g
(c) Determine the empirical formula of the hydrocarbon.
moles C = 2.40 / 12.01 ≈ 0.200 mol
moles H = 0.403 / 1.008 ≈ 0.400 mol
ratio H:C = 0.400 / 0.200 = 2 → CH₂
But CH₂ isn’t stable — multiply by 2 → C₂H₄
Empirical formula: C₂H₄
(d) If the molar mass of the compound is 56.1 g/mol, what is its molecular formula?
empirical mass = 28.05 g/mol
n = 56.1 / 28.05 ≈ 2 → molecular formula = C₄H₈
Answer: C₄H₈
(e) Is this compound likely ionic or covalent? Justify your answer based on bonding and physical properties.
This compound is covalent because it is composed only of carbon and hydrogen (nonmetals), forms molecules, and has low melting/boiling points typical of organic compounds. Ionic compounds usually involve metals and nonmetals and form crystalline solids with high melting points.
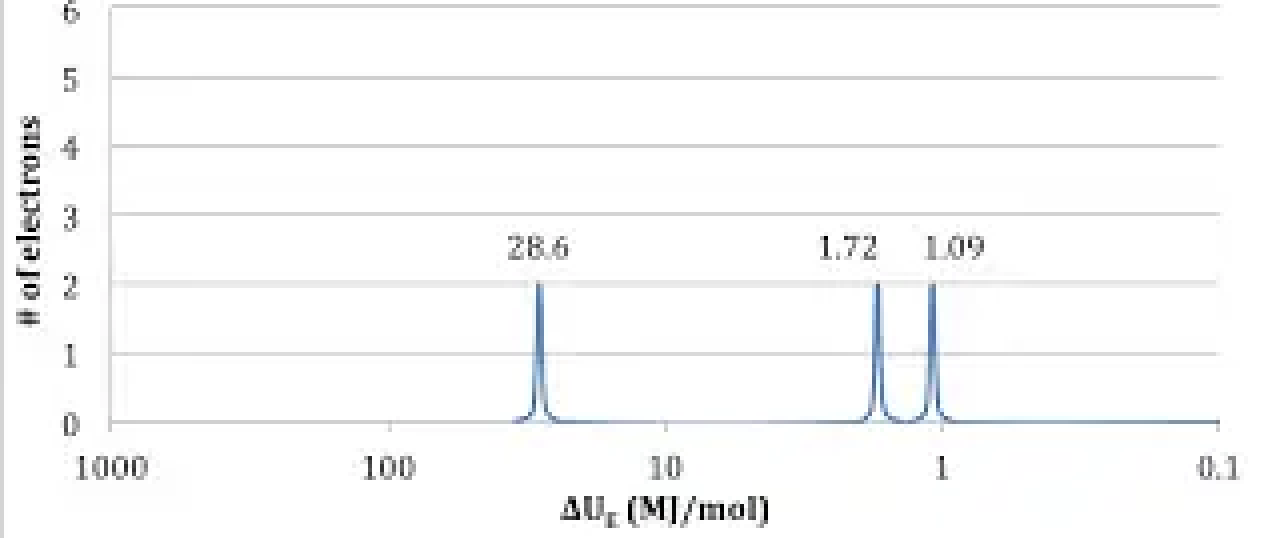
The photoelectron spectrum of an element shows three peaks with binding energies and relative electron counts as follows:
(a) Identify the element and justify your answer using the data.
(b) Would the peak corresponding to the 2p subshell be to the left or right of the 2s peak on the spectrum? Explain.
(a) Identify the element and justify your answer using the data.
Total electrons = 2 + 2 + 2 = 6
Element with 6 electrons is carbon (C)
Answer: Carbon
(b) Would the peak corresponding to the 2p subshell be to the left or right of the 2s peak on the spectrum? Explain.
In a PES spectrum, binding energy increases from left to right. The 2p electrons have slightly lower binding energy than 2s electrons due to less penetration. So the 2p peak would be to the left of the 2s peak.
Consider the elements Na, Mg, Al, and Si.
(a) Explain the general trend in first ionization energy across this period.
(b) Identify the exception to the trend and explain why it occurs.
(c) Which of these elements is most likely to form a +3 ion? Justify your answer.
(a) Explain the general trend in first ionization energy across this period.
First ionization energy generally increases from left to right across a period due to increasing nuclear charge and decreasing atomic radius, which hold valence electrons more tightly.
(b) Identify the exception to the trend and explain why it occurs.
The exception is that Al has a lower ionization energy than Mg because Mg has an electron configuration ending in 3s² (full s-subshell), which is stable. Al ends in 3s²3p¹, and the p-electron is easier to remove because it is higher in energy and shielded by the s-electrons.
(c) Which of these elements is most likely to form a +3 ion? Justify your answer.
Aluminum (Al) is most likely to form a +3 ion because it loses its three valence electrons (3s²3p¹) to achieve a noble gas configuration ([Ne]).