Chem H Sem. 1
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
law of conservation of mass/energy
mass: matter is not created nor destroyed in any chemical or physical change
energy: energy can neither be created nor destroyed
chemical vs physical
Physical: atoms DO NOT change their fundamental associations, different form but same substance easily reversed, solid to liquid to gas, dissolving,
Chemical: atoms DO change fundamental association, whole new substance is formed, combustible, rusts, tarnishes, four indicators:
1. unexpected color change
2. energy change: form of heat, light, sound
3. gas inolved, indicated by see bubbles, smell something vapor around air and stuff, hear fizzing, could have explosion
4. precipitation (?)
Tyndall effect
Answers is this solution, suspension, or colloid? Uses a laser to determine how big the particle size are based on if lights reflected or not
- Positive tyndall effect: see laser beam, big enough particle size, heterogeneous
- Negative Tyndall effect: don't see laser, really small particle size, homogeneous
observation, hypothesis, theory, scientific law
observation: quantitative (numbers) and qualitative (senses)
hypothesis: testable assumption to explain an observation (WHY? for one experiment)
scientific theory: comprehensive idea that is well tested, aiming to be disproved (WHY? for many experiments)
scientific law: a brief statement that synthesizes observations (tells us WHAT? happens but not why) can only be true if many many observations are taken into account
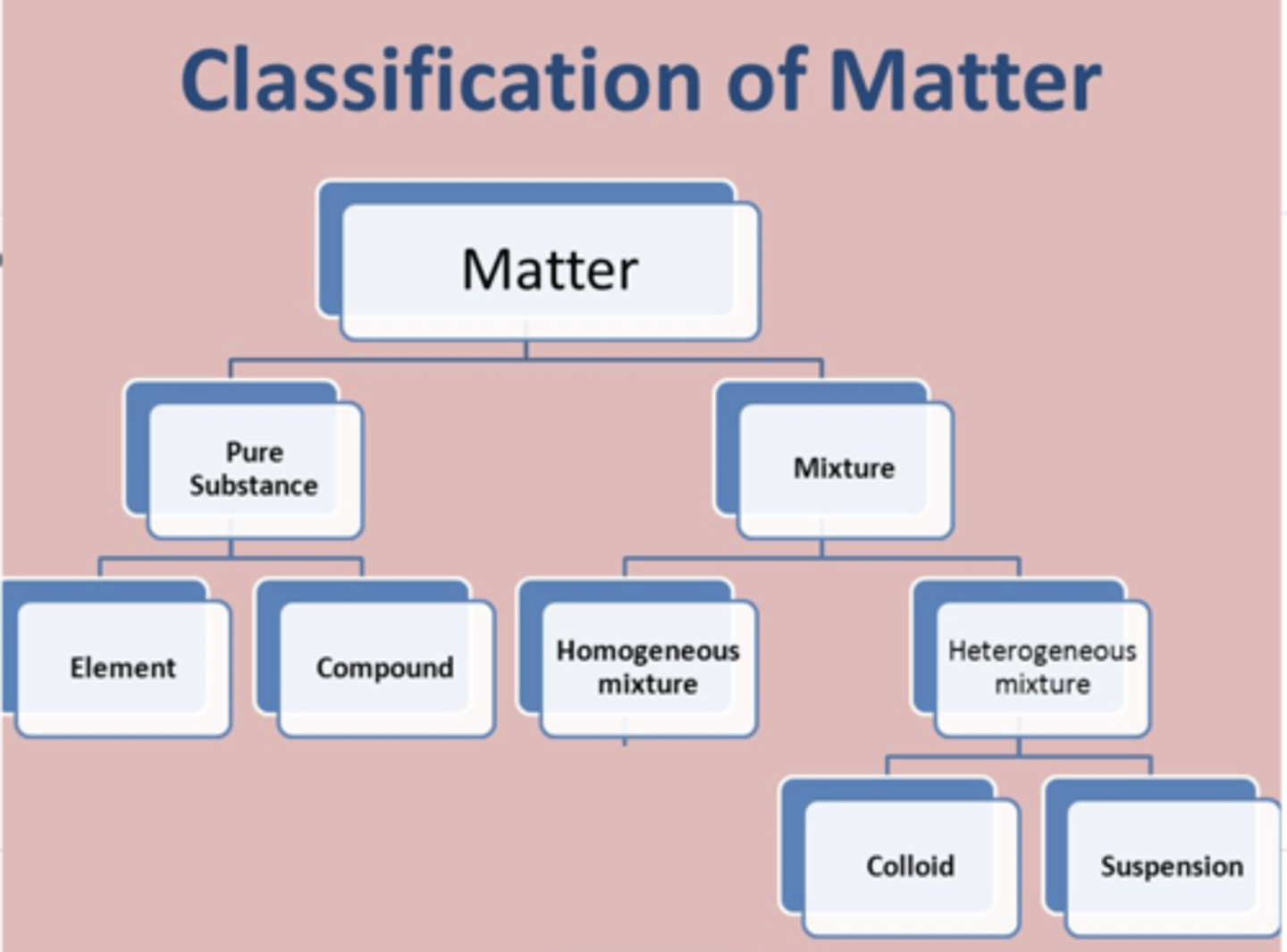
How do we classify matter
MATTER
pure substances: definite composition; every one particle has definite ratio of elements in a compound or just of the element; composed of one kind of particle; can only be separated by chemical means
element
compound
mixtures: composed of more than one kind of particle; can be broken down by physical means; saltwater mixture could be any percent water or salt so its variable composition
homogenous: aka solution, SMALLEST particle uniform throughout
heterogeneous: not uniform, you can pick stuff out
suspensions: LARGEST particle muddy water, if you let it sit it will settle out its two parts to be easily diferentiated
colloids: MEDIUM particle mixed throughout and will not settle out, looking uniform even tho particles can be picked out
accuracy, precision, percent error
accuracy: values that you determine are close to accepted value
precision: close to each other on values but not necessarily close to the accepted
percent error: error is determined by finding the difference between your experimental value (what you got) and the accepted value (what you should have gotten). This is always a positive value
also random but y-axis: whatever we want to be in numerator of our measurement so MASS
x-axis: volume
bc m/v=d
sig fig rules
1) non-zero digits are always significant
2) zeros:
a. leading zeros are not sig
b. captive zeros are sig
c. trailing zeros are sig
3) exact numbers (determined by counting not measuring) have infinite sig figs
- measuring: go one decimal over what is known
- multiplication/division: round final answer so that it has same sig figs as measurement with least number of sig figs
- addition/subtraction: round final answer so it has same number of decimal places as the measurement with least number of decimal places
intensive vs extensive properties
intensive: property is independent of amount, density is same no matter what amount (EX: density, melting point, boiling point, specific heat) good for identifying material
extensive: does depend on amount (EX mass, volume, length)
y axis vs x axis
particle diagram requirements
Different Substances: Use different shapes to represent different substances or types of particles.
Density Representation: Ensure that the spacing between particles reflects differences in density—denser substances should have particles closer together.
Conservation of Matter: When showing a chemical reaction, ensure that the number of particles before and after the reaction remains the same (to reflect the law of conservation of mass—no matter is created or destroyed).
Chemical Bonds: If particles are chemically bonded, depict them as connected or linked shapes.
properties of solids, liquids, and gases
- matter never stops moving
Solids: form of matter that has its own definite shape and volume, dancing molecules less with molecules energy bouncing off eachother rather than past
Liquids: form of matter that flows, has constant volume, and takes the shape of its container, molecules moving fast enough to go past eachother with their force of attraction not strong enough to stay solid
Gas: form of matter that not only flows to conform to the shape of its container but also fills the entire volume. with evaporation, when water is liquid there is complete disorder within molecules. the molecules at the top forming surface, one molecule can pop out and escape into air now turning into vapor. warmer water -> more jostling amongst molecules -> more pop out into the air. condensation is when gas water molecules touch surface, they get colder and start moving slower turning into water.
law of thermodynamics + endothermic vs exothermic
energy cannot be created or destroyed, only transformed
endothermic: when system contains more energy than surrounding
exothermic: surroundings have higher energy (it's going into the system)
temperature change and pahse change formula
MCDELETA TJ LJFS:IODHLFK
frequency vs wavelength + high->low energy rank
as frequency increases, wavelength decreases, and energy increases
wavelength: the distance between adjacent wave crests
frequency: number of wave cycles that pass a given point per second
high->low energy rank:
gamma—x-rays—UV—vis light—infrared—microwaves—FM—AM radio
Bohrs model vs quantum mechanic model (heisenberg uncertaintiy principle)
Bohrs model: (based on rutherford) electrons move in discrete and stationary circular orbitting distance from the nucleus thats in the center (only explains for hydrogen atoms)
quantum mechanic model (schroedinger): electrons are noto found in fixed orbtis but rather round in probability regions called orbitals with diff shapes (spdf)
heisenberg uncertainty principle: you cant know both the speed or location of an electron
color on a particle level
solution absorbs color opposite of what it reflects. the energy excites the electrons who go up and down the orbits at a certain speed. an increase of speed move towards purple and decrease speed moves towards red
orbital stuff and locations on periodic table (n, l, ml, ms)
n: prinicple quantum number aka energy level and how far away atom is from nucleus
l: orbital (s,p,d,f), each number gets a shape, whatever n is, l is 0 to n - 1 the range l could be so n ≠ l
ml: orientation of orbital, range of -l to l
ms: spin
l = 0 --> s (1 box, 2 electron each so 2 columns long)
l = 1 --> p (3 boxes, 2 electrons each so 6 columns long)
l = 2 --> d (5 boxes, 2 electrons each)
l = 3 --> f (7 boxes, 2 electrons each)
EXCEPTIONS TO NAMING are Cr, Cu, Mo, and Ag because s and d would rather be half full of full so the s moves to become half full to make d 5 so its full
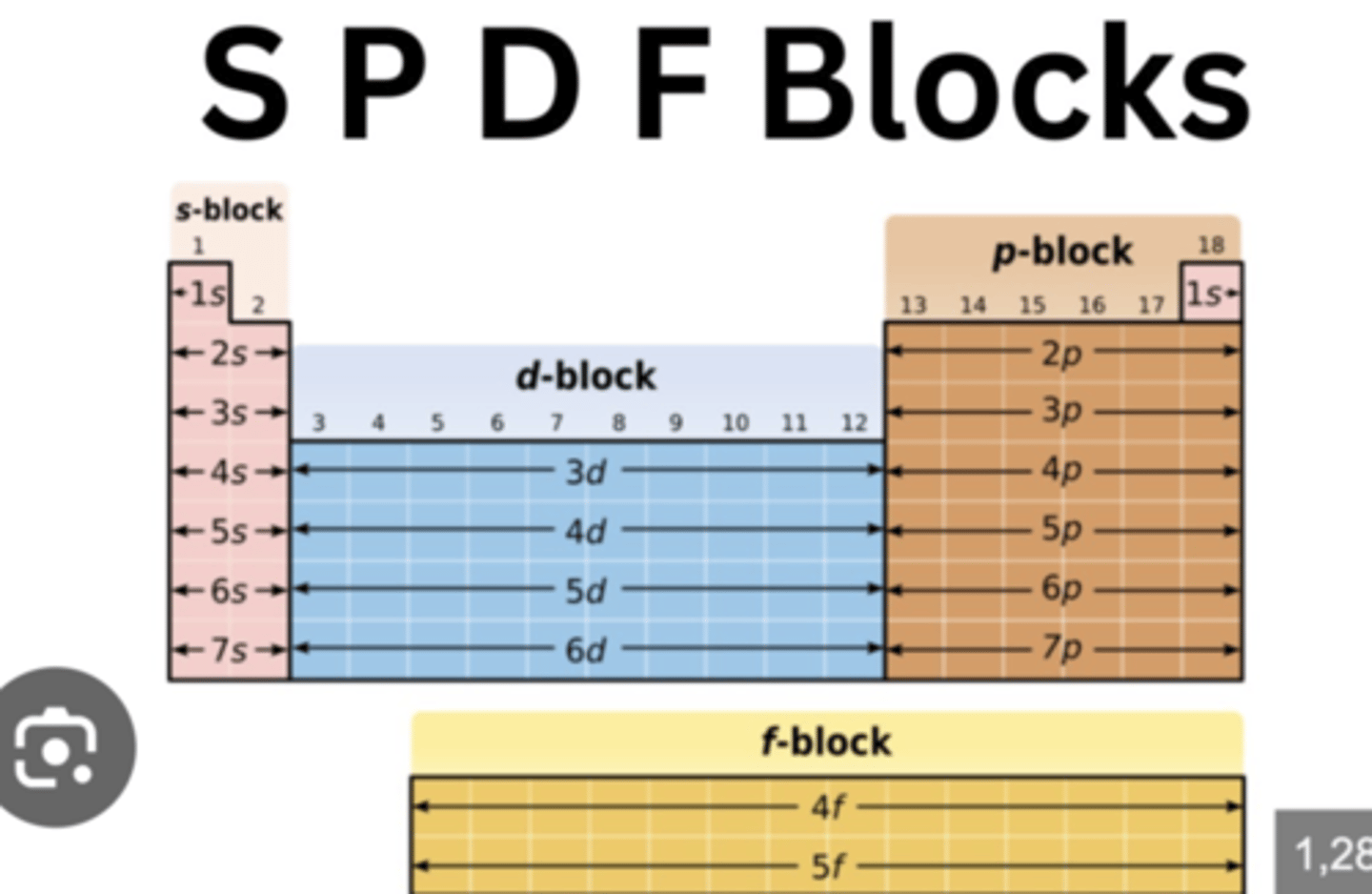
group locations on periodic table
halogens, nobles gases, alkali metals, alkaline earth metals, metals/nonmetal/metalloids
ignore bottom part we don't have to know that but metalloids are like "ones touching the staircase" but thats such cap bc Al is discluded and At and Ts and Og
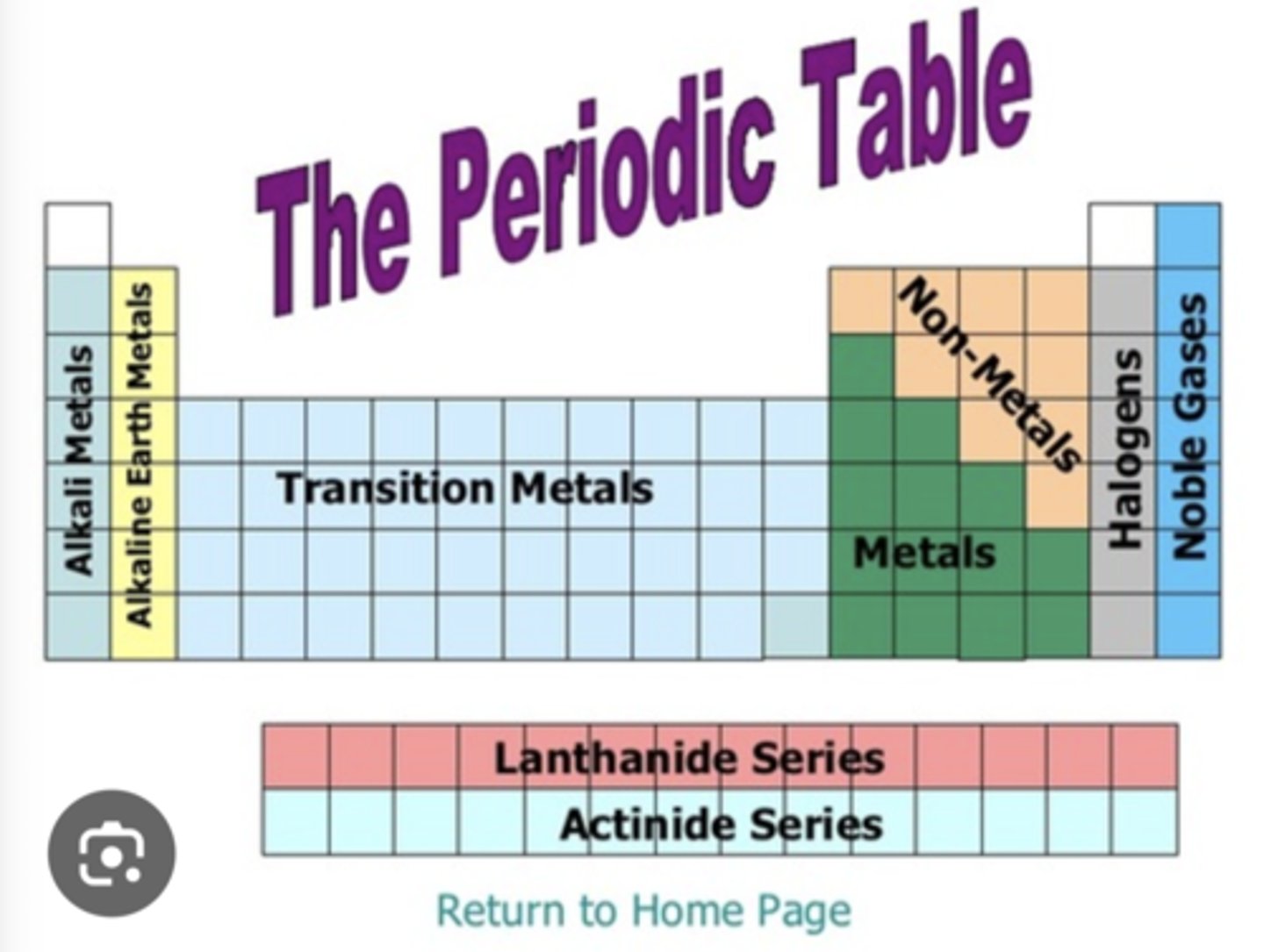
element for atomic size, ionization energy, reactivity
atomic size - Francium (Fr) the size of an atom
ionization energy - Helium (He) the amount of energy needed to remove an electron from a neutral atom. (EXCEPTIONS: it's easier to remove electrons for unstable elements like B, O, Al, and S so thier IE is a little less)
electronegativity - Fluorine (F) measure of an atoms ability to attract electrons
reactivity - Francium (Fr) the bigger the atom is, the more reactive it is because it's more likely to lose or gain electrons and react with others (except chat says otherwise so do we even have to know this)
anion vs cation
anion: an anion is a negatively charged ion and its formed when an atom gains an electrons
cation: a cation is a positivley charged ion formed when atom loses electron
effective nuclear charge
basically a measure of how strongly the atom is getting pulled toward the nucleus determined by the ratio of proton to valence electron ratio. higher ENC means more protons than electrons, so stronger pull. lower ENC means less protons in general, so weaker pull.
Democritus, Plato + Aristotle, Alchemists (do we have to know this erm), Lavoisier
Democritus: first scientist to talk about what things were made of. He thought everything was made up of these tiny, indivisible particles called "atoms"
Plato & Aristotle: They refuted that, thought there were 4 components of what things were made up of (air,water,earth,fire) These ideas faded
Alchemists: Next ppl to talk about how things were made, wanted gold and elixrs of life, like Isaac Newton in 1700s (okay and)
Lavoisier: law of conservation of mass, father of chemistry, first person to take this info and write a textbook
Dalton's Atomic Theory (1808) (+ law of definite/multiple proportions
1. (FALSE) All matter is composed of tiny, indivisible particles called atoms (atoms are divisible)
2. (FALSE) All atoms of a given element are identical (isotopes exist)
3. (TRUE) Atoms combine in whole number ratios to form compounds (aka Law of definition proportions (Proust): atoms of the same chemical compound all contain the same element-to-element ratio of the elements within the compound.)
4. (TRUE) Atoms separate, join, and rearrange in a chemical reaction (aka multiple proportions law (Dalton):The same two elements can form different compounds based on their ratio.)
JJ Thompson's Cathode Ray Tube Experiment (1898)
JJ Thompson discovered electron by using the cathode ray tube:- he put argon gas through the cathode ray tube, and there was a negative magnet in the middle. All the electrons reflected off the negative side. He concluded if they're bouncing off the negative, they must be negative
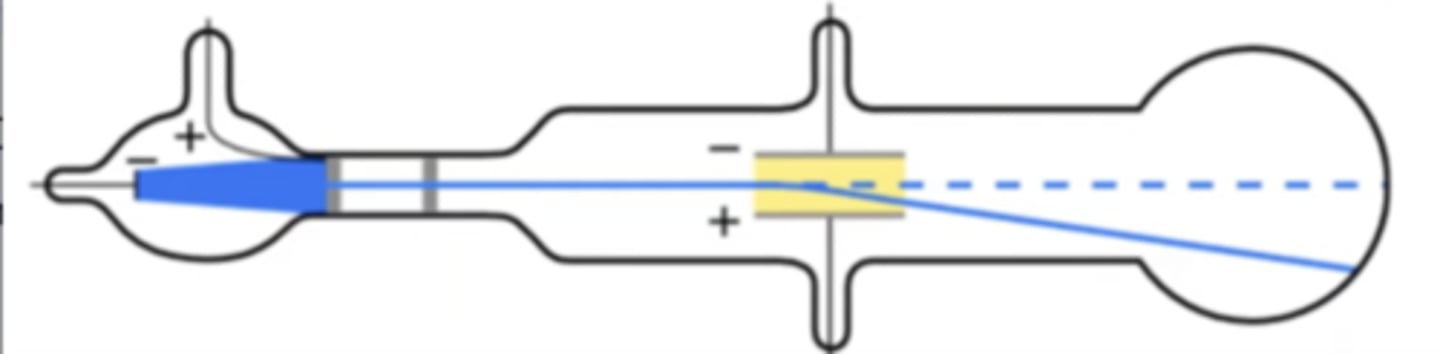
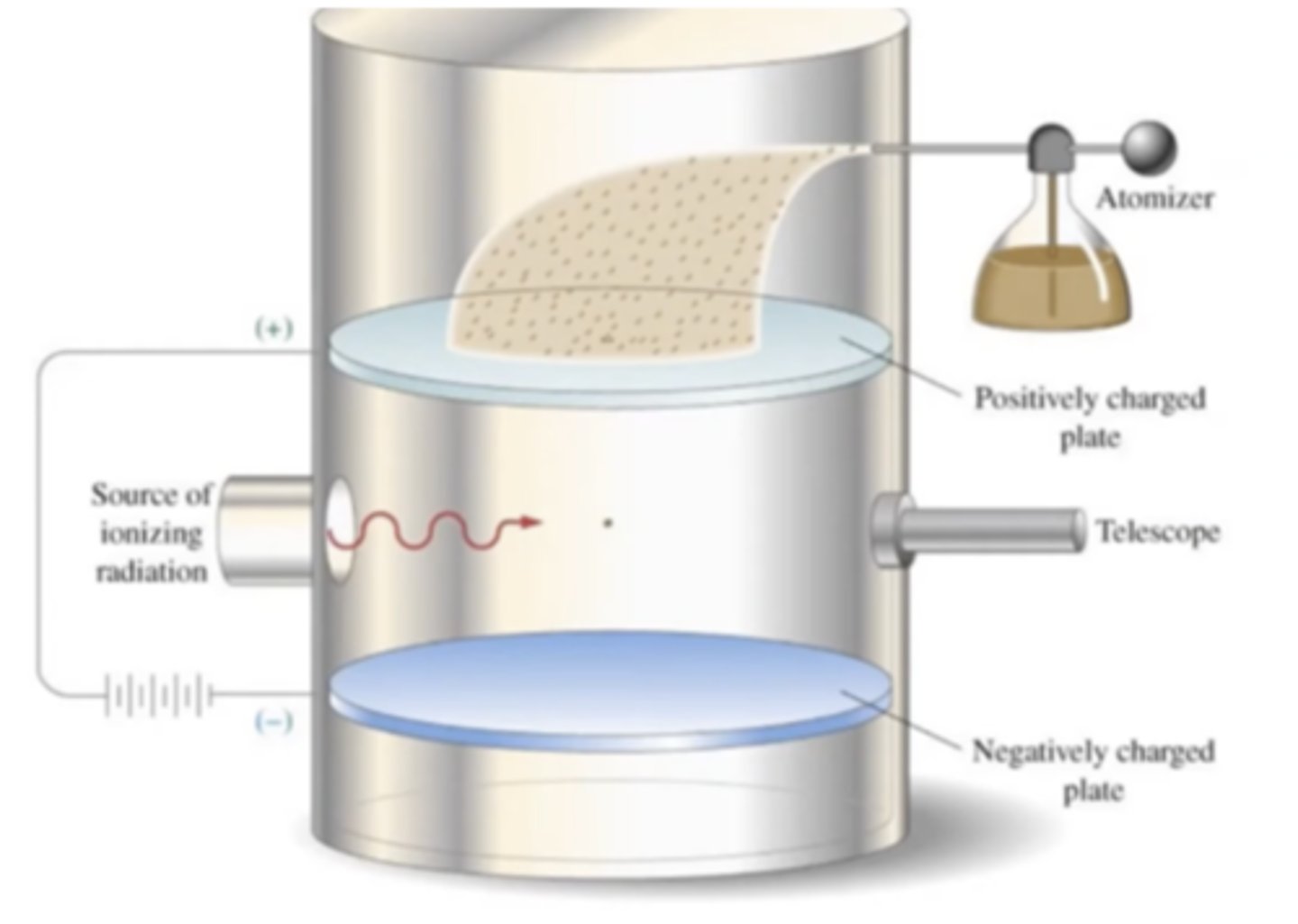
Millikan's Oil Drop Experiment (1909)
he separated an atom of oil and figured out how much energy it takes to float it and figured that was the mass.discovered mass of protons and electrons. he separated one atom of oil
mass/amount relationship of protons, neutrons, electrons
Protons and neutrons have very similar masses and electrons have almost negligible mass (if proton/neutron was mass of baseball, electron is grain of rice) but the amount of protons = amount of electrons
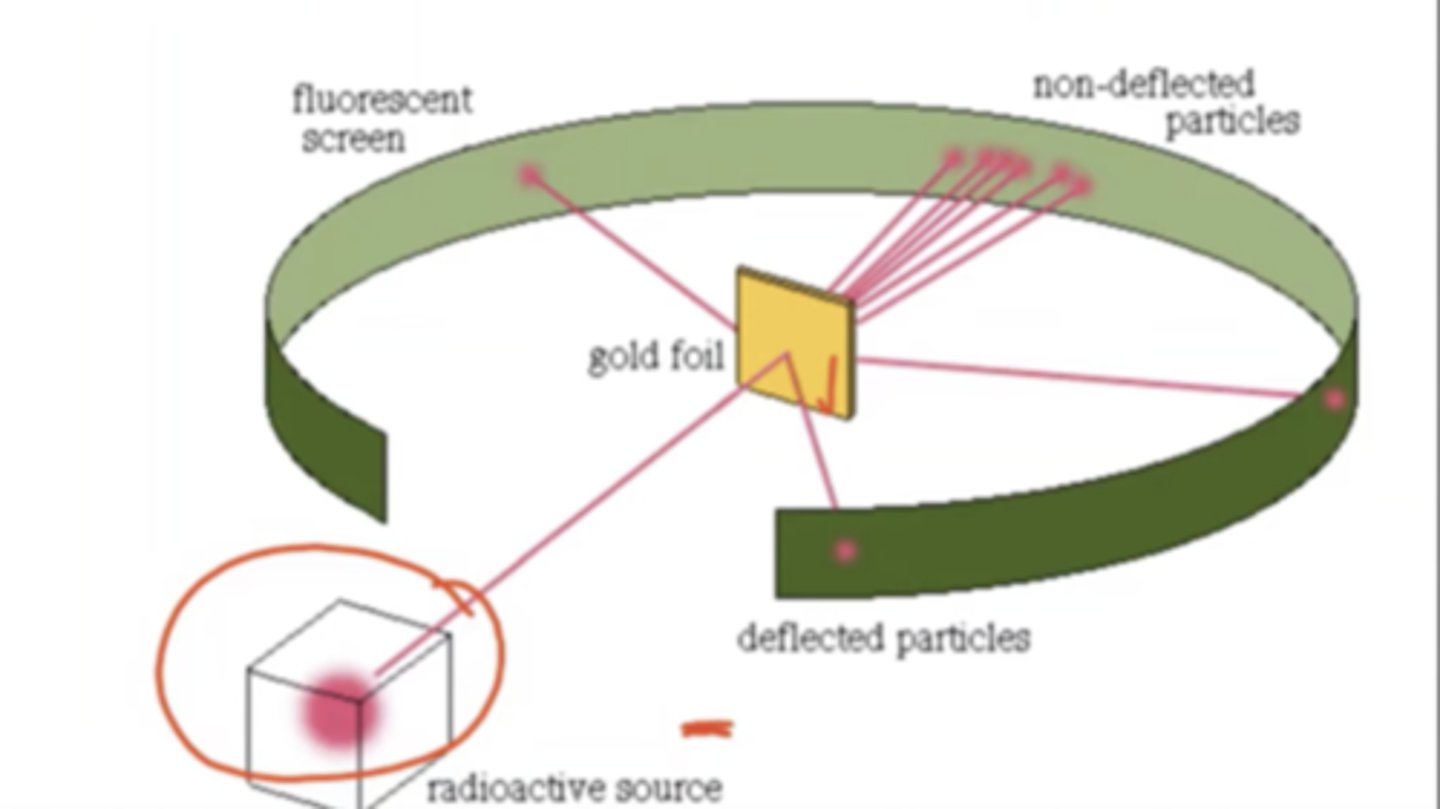
Ernest Rutherford's Gold Foil Experiment
Ernest Rutherford's Gold Foil Experiment
He had the gold foil, and shot alpha particles at the foil. 98% went thru, 2% deflected because it was hitting the nucleus of the atoms, thus he discovered there was a nucleus theree
Q: Is thompsons stupid plum pudding model correct atom structure
Hypothesis: If it's correct, alpha particles would go straight thru
Results: 98% went through, 2% deflected
Conclusion:
1) atoms are mainly empty space
2) atoms have a small, massive center
Goldstein (1886)
discovered proton after Thompson's cathode ray tube experiment and the discovery of the electron because they figured the atom had to be neutral
Chadwick (1932)
discovered the neutron by doing similar experiment has rutherford but instead of gold he used a burrillium target
How are isotopes related to the atomic mass of an element?
atomic mass is the average of all isotopes naturally occurring for that elementALWAYS REPORT ATOMIC MASS 2 DECIMAL PLACES WITH UNIT AS amu)
Radiation, radioisotope, radioactive decay, radioactive ALSSOOO Where does radiation come from? Radioactive decay (+ its nature)?
Radiation: energy as an electromagnetic wave (form of energy), or moving subatomic particles
Radioisotope: any radioactive isotope, same number of protons and different number of neutrons
Radioactive Decay: nucleus unstable, decays by losign energy by emitting radiation
Radioactive: describes a substance that emits tiny, invisible, energetic particles from the nuclei of its compound atoms
where radiation and radioactive decay comes from + RD's nature: both come from nucleus, radioactive decay has a random nature. there's a possibility an atom might decay, but it's random which do.Moreover, radioactive isotopes decay by emitting radiation. If it is unstable, it is going to decay by emitting radiation. Radiation comes from the nucleusElements possible for radioactive decay are ones with a heavy mass and neutron rich
alpha decay, beta decay, gamma emission, positron emission
alpha decay: large in size, low in penetrating power (blocked by paper) and can't pass through easily 4/2He
beta decay: medium size, medium penetrating power, (blocked with lead/some plastic) -1e
gamma emission: composition of energy not effecting mass, small size and high penetrating power (difficult to block even lead) 0γ
positron emission: medium size medium power +1/0
fission vs. fusion
fission: whne you have a large atom that breaks into 2 smaller ones. Produces LOTS of energy but dangerous (still used for nuclear power plants). For fission, you take the nucleus and bombard it with a nuetron, which breaks it apart. When it breaks, it makes these 2 smaller fission produces and more neutrons! This starts chain reaction.
fusion: when 2 smaller particles combine to form a heavier nucleus. a little safer than fission but the only problem is youo only get the energy if you apply unsustainably high temps
people and experiments recap in order
Democratus - tiny, indibisble particles
Lavoiser - law of conservation of mass, father, textbook
proust - law of definite proportions
Dalton - theory
JJ Thompson - electron
Goldstein - proton
MIlikan - mass of protons and electrons
Rutherford - atoms have small, massive center
Chadwick - neutron
Ionic vs covalent naming
Ionic: metal gives electron to nonmetal
- opposite sides of the table, METAL + NONMETAL (with ide ending)
- for transition metals, roman numebrs indicate metal charge
- Subscript tells you number of atoms
- can reduce subscripts
from lab: conductive, don't melt
Covalent: only nonmetals, so right side of periodic table
- NONMETAL + NONMETAL
- octet rule (8 for all), duet rule (2 for H)
- Left of C fine with less (B)
- Right of carbon fine with more (P, S, Cl)
- in diagram, most electronegative john goes in center
DONT REDUCE SUBSCRIPTS
- prefixes are mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca,
from lab: partial conductors, melt
Naming acid rules
binary acids: an element (nonmetal) bonded with hydrogen
hydro-____ic acid
oxyacid: polyatommic + oxygen + element + hydrogen
ate --> ic ite --> ous
+ acid
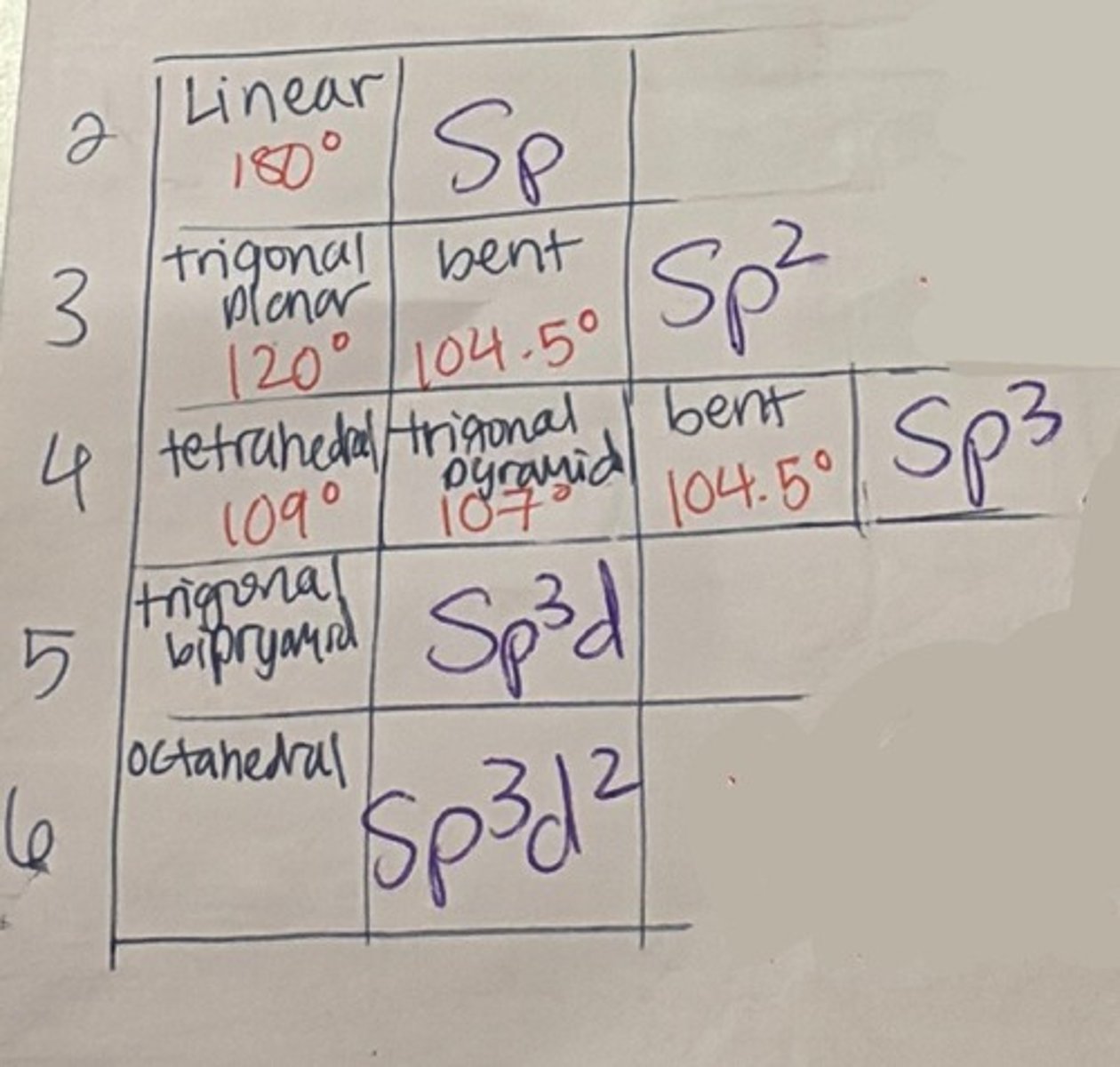
electronic vs molecular geometry
Electronic Geometry: how many things connected to central atom: refers to the position of all electrons in a molecule, bonding or no bonding
Molecular Geometry: takes into account lone pairs: the position of only the bonding pairs of electrons in a molecule
- when there's no lone pairs, EG = MG
ALSO
single bond - sigma
double bond - sigma and pi
triple bond - sigma and 2 pi
random note: polar only dissolves polar and NP dissolves NP
polarity of bond vs molecule
polarity of bond is determined by electronegativity. if the bond is polar, then the entire molecule can either be nonpolar (if its symmetric) or polar (if its NOT symmetrical aka assymetrical). If the bond is non polar, the molecule has to be nonpolar.
- with polar bonds, you draw the plus arrow thing and it points to the element that is more electroengative and the element that everyones pointing at is S-
- higher electronegativity = more polar elementA
- for linear molecules, if its the same element twice its symettrical if its two diff elements its assymetrical
