Bridge to A level
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Give three ways in which the plum pudding model differs from the current model
The current model included a nucleus containing protons and neutrons '
No energy level
Most of the atom are empty space
Why do we use the Bohr Model
It is simple and clear to understand and it still supports and explains trends
Atomic number
Number of protons in the nucleus
Mass number
The total number of protons and neutrons in the nucleus
Isotopes
Atoms of the same element with the same number of protons but different number of neutrons
Why do isotopes have the same chemical properties?
They have the same electron configuration and the same number of electrons
Which physical properties of isotopes might be different?
The mass, Density, Boiling point, Diffusion rate
Relative Isotopic mass
The mass of an isotope compared with 1/12th mass of an atom of Carbon-12
Empirical Formula Calculation
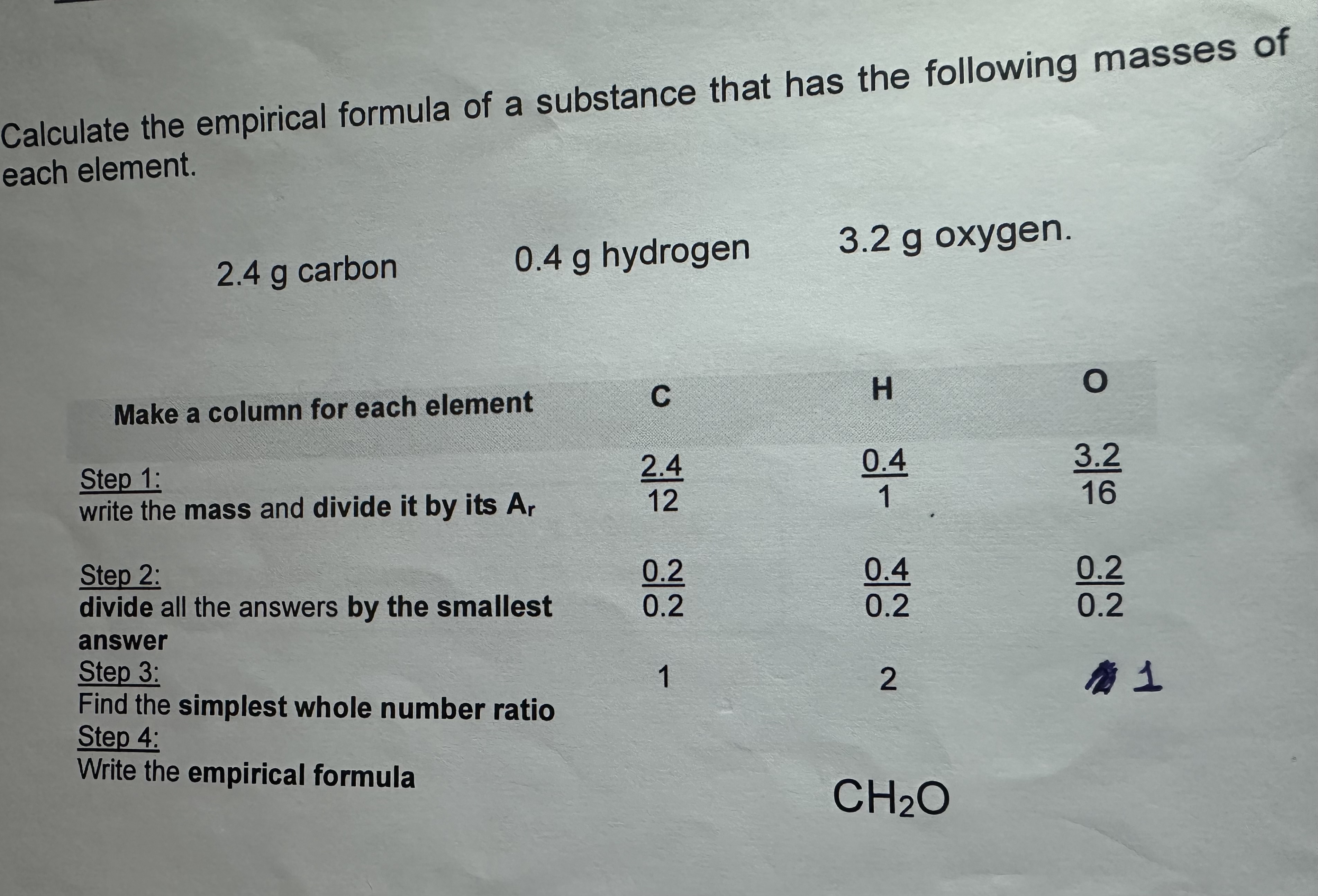
Calculating the Molecular formula from the Empirical Formula

Water of Crystallisation

Suggest a modification that a student could make to be confident that all of an element has been heated in water of crystallisation
Heat to constant mass