L4: Synapse formation
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
73 Terms
The assembly of neural circuits can be subdivided into three phases
Axonal pathfinding→
the projection of axons into the vicinity of poast-synaptic targets
Target recognition→
cessation of axonal growth upon contact with its postsynaptic targets
Synaptogenesis→
conversion of growth cone→ specialised pre-synaptic strucutures
induction of precisely aligned postsynaptic specialisations in the target cell
Discovery of synapse
syn→ together
haptein→ to clasp
found due to lag time
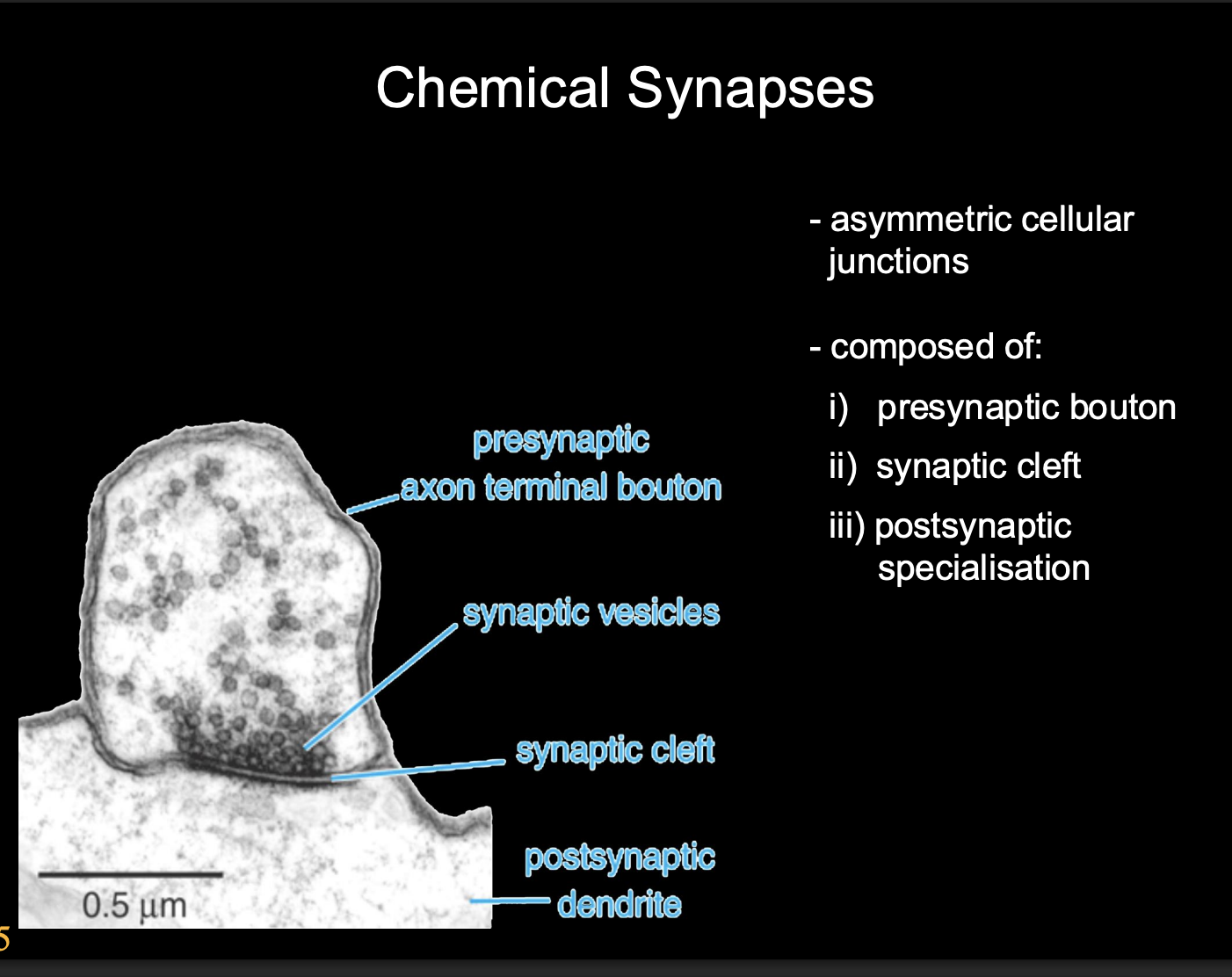
Features of chemical synapses
Asymmetric cellular junctions
Composed of
Presynaptic bouton
synaptic cleft
Postsynaptic specialisation
control and define turning how many vesciles are released
depends on the sensitivity of the post synaptic neuron
relay station
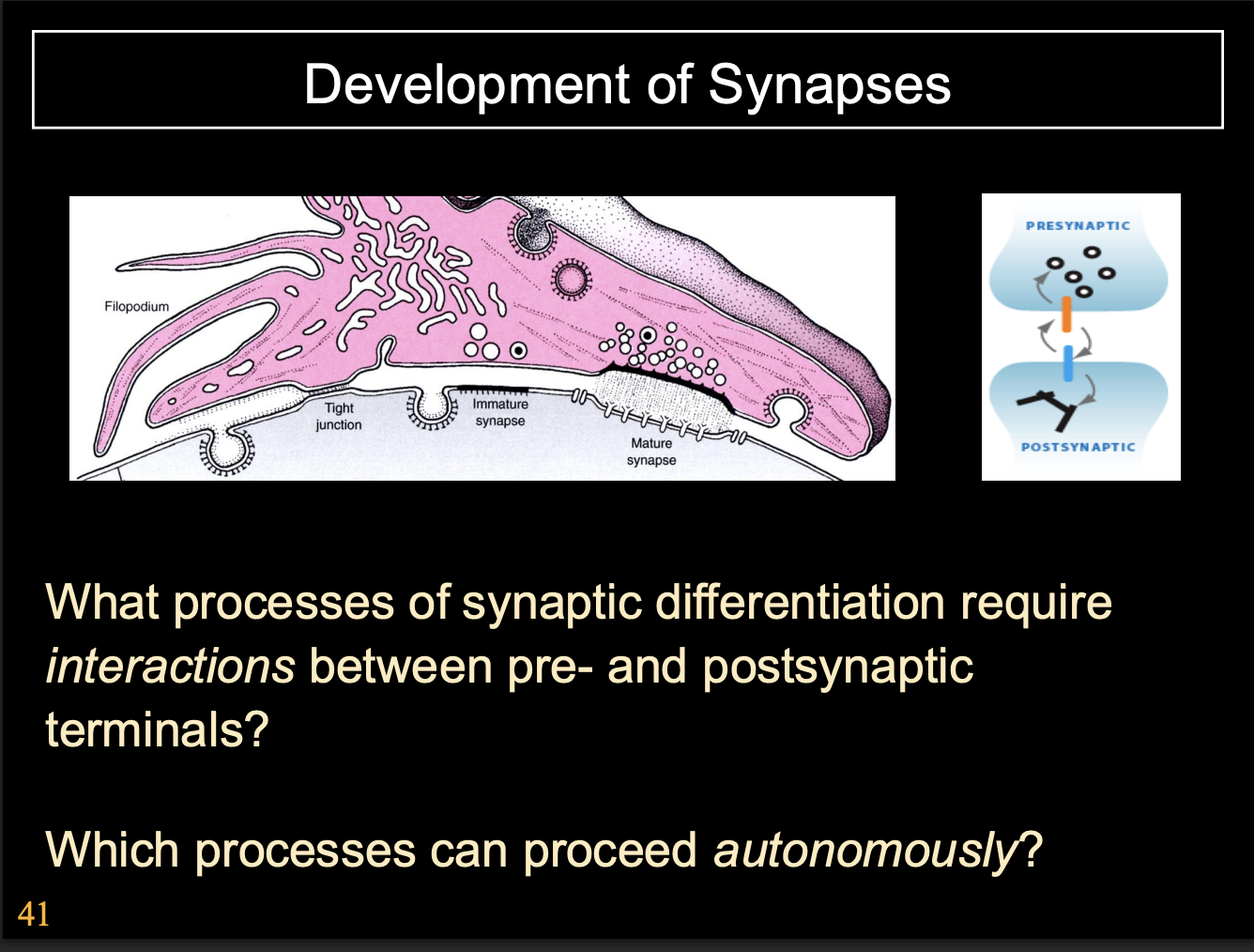
How do synapses form
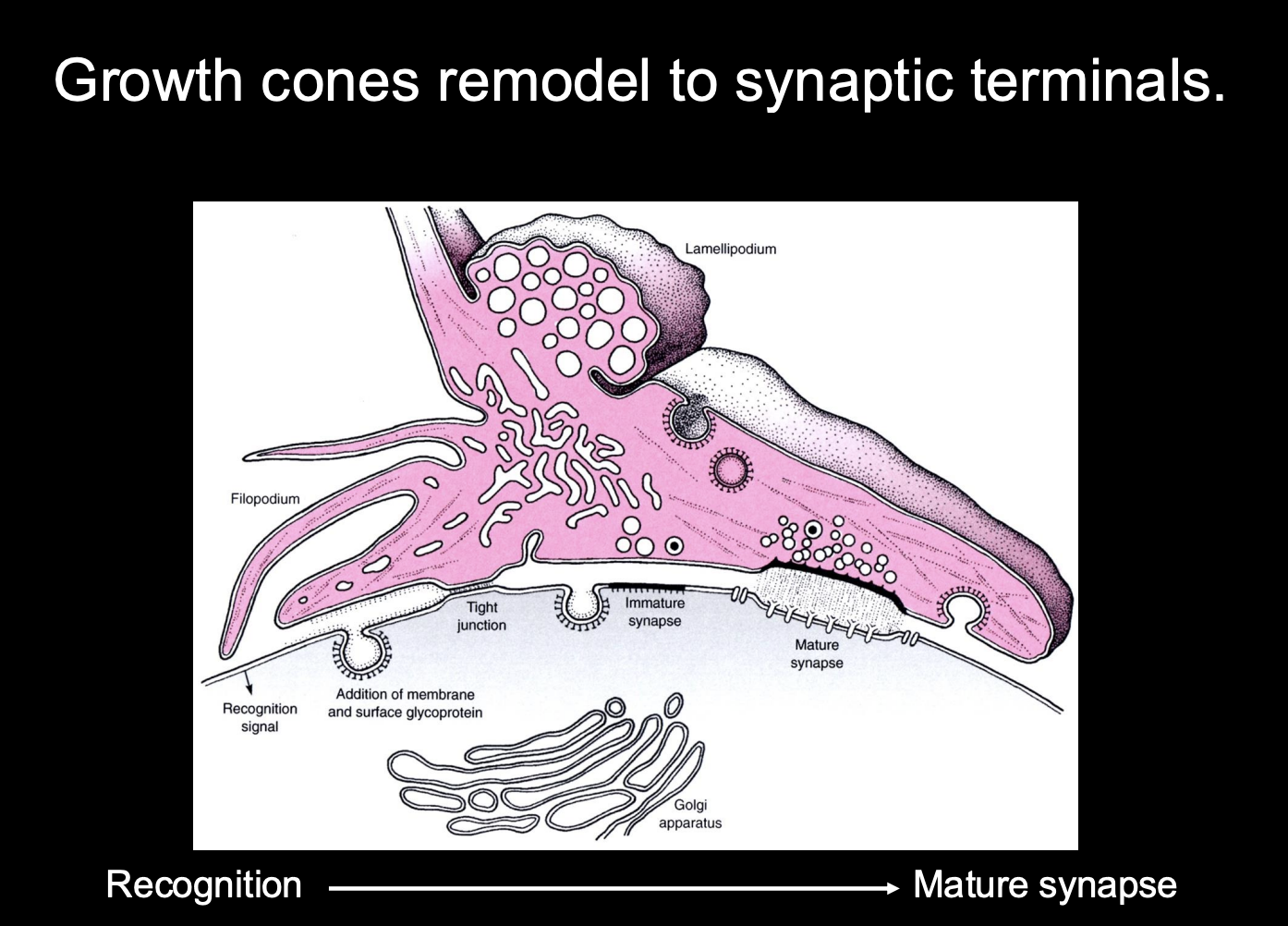
Growth cones remodel into synaptic terminals
How:
recognise the site→ mature into synapse
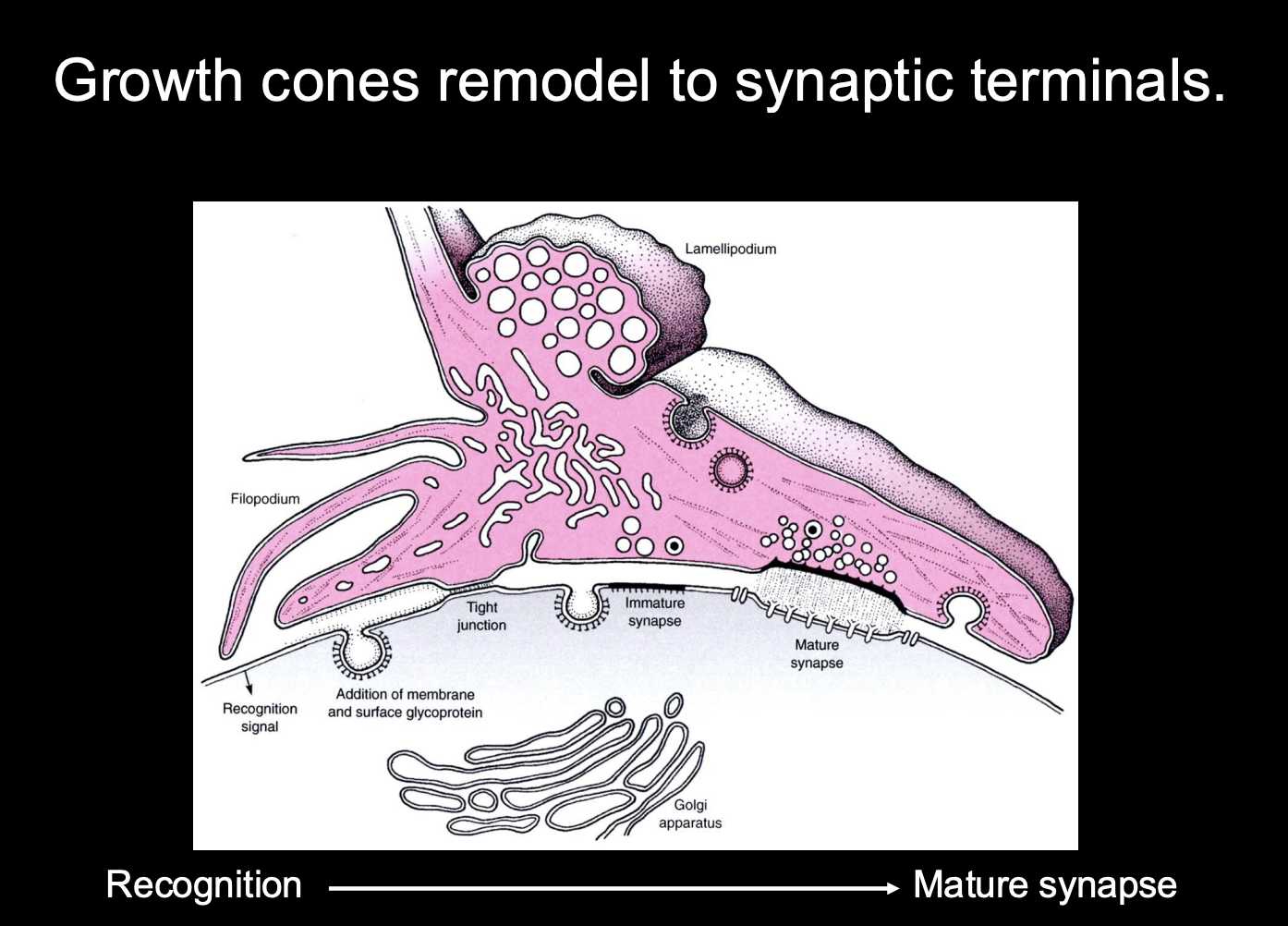
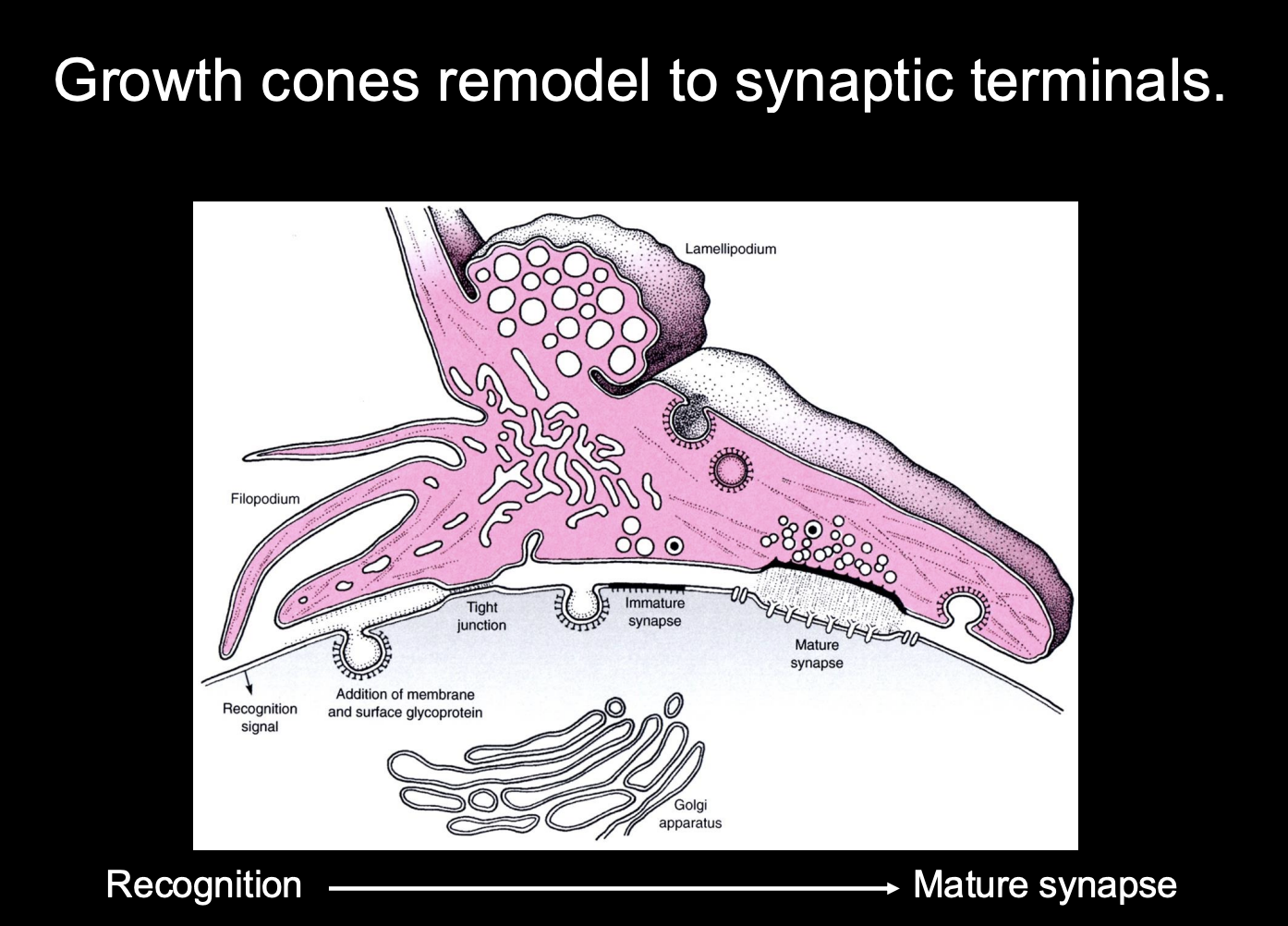
Development of Synapses→ at the presynaptic neuron
Presynaptic
Growth cone slows down on approaching targets
Filopodial explorations recede and growth cone changes to bouton-like shape
Growth cone is aligned with postsynaptic specialisations
Vesicle release machinery assembles
Growth cone can release neurotransmitter in response to electrical stimulation
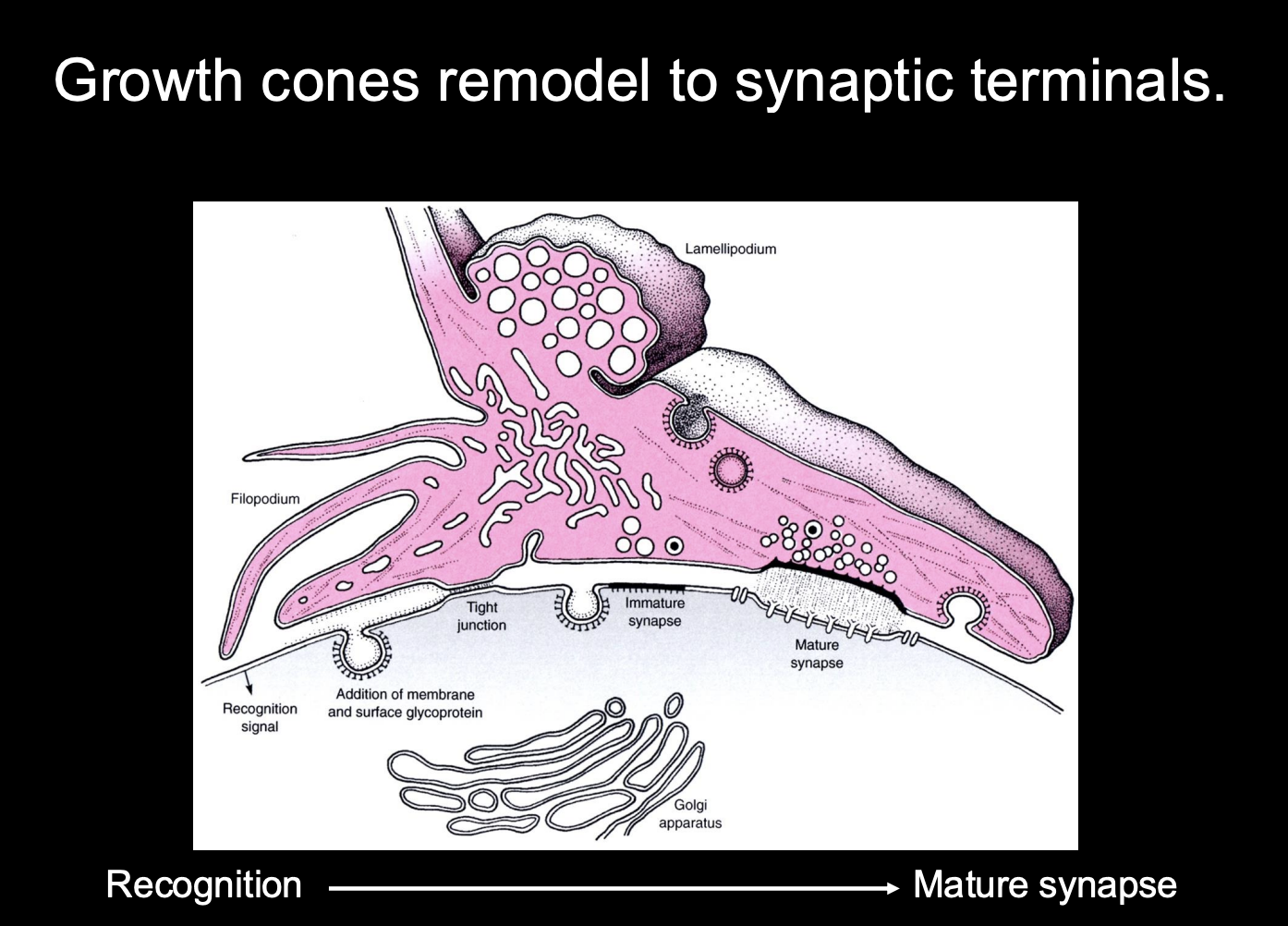
Development of Synapses→ at the post-synaptic neuron
Postsynaptic
Neurotransmitter receptors cluster opposite presynaptic terminal
extra-synaptic transmitter receptors are removed
cytoskeletal specialisations form apposed to presynaptic terminal
e.g electron dense matrix
Overview of the synaptic development→ what is required
conversation between the pre and post synaptic neurone
results in precisely aligned, highly specialised pre ad post synaptic sites
However, these processes can either be:
Autonomous→ just always happens
Come processes require interactions between pre and post synaptic terminals
next question to ask
which processes are which
Why is the neuromuscular junction (NMJ) an invaluable model system
easy access for manipulation
easy access for observations
large size
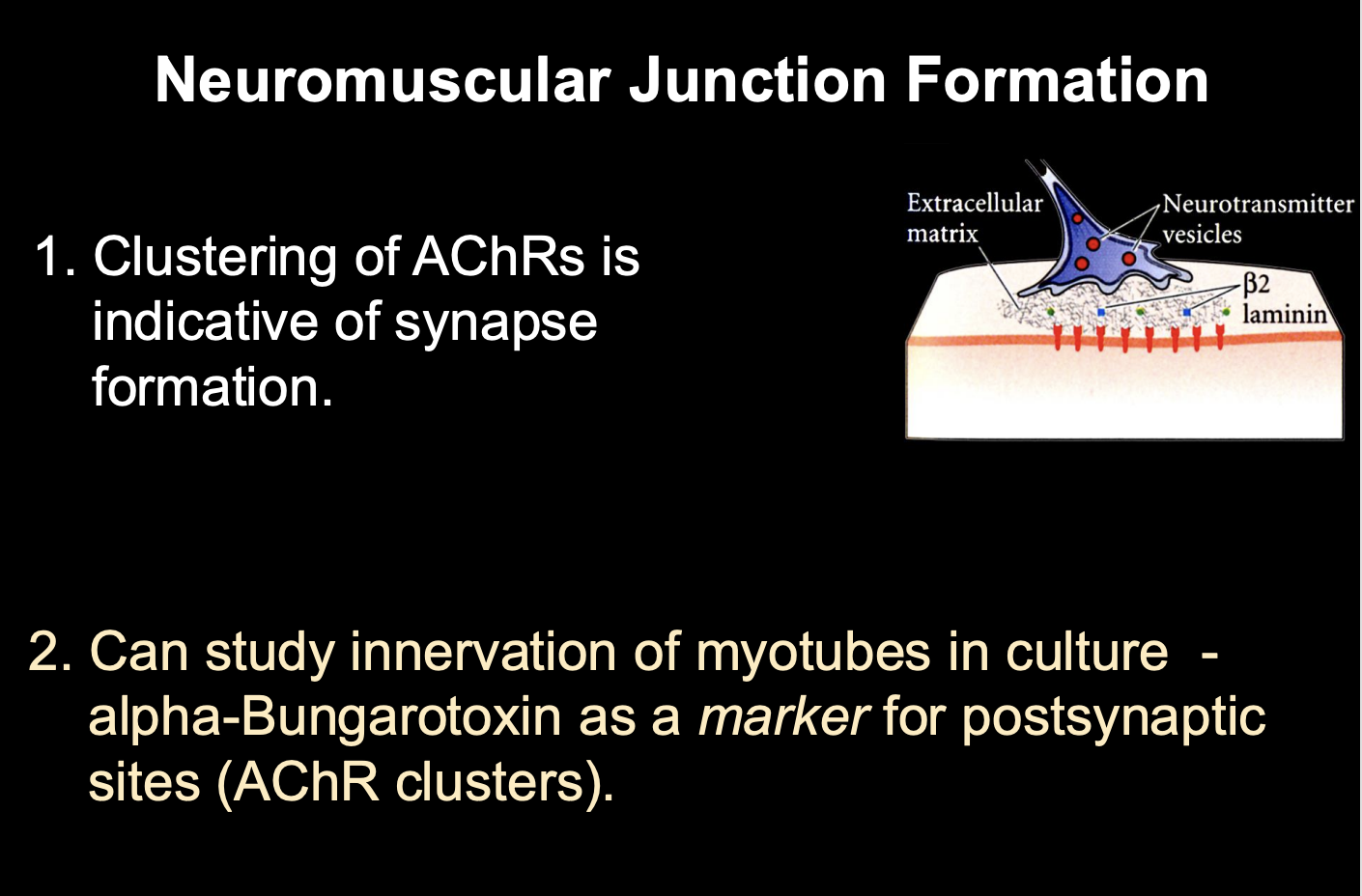
What did observations of synaptogenesis at the vertebrate NMJ show
Clustering of AChRs is indicative of synapse formation
as the motoneuron growth cone arrives at the target, AChRs begin to aggregate at the site of innervation (presynaptive synaptic site)
How was this shown
Use alpha bungarotoxin as a marker for postsynaptic sites
binds irreversibly to AChR
bound with a fluorescent tag
Shows→ growth cones arrive and aggregate into clusters→ forming a synapse
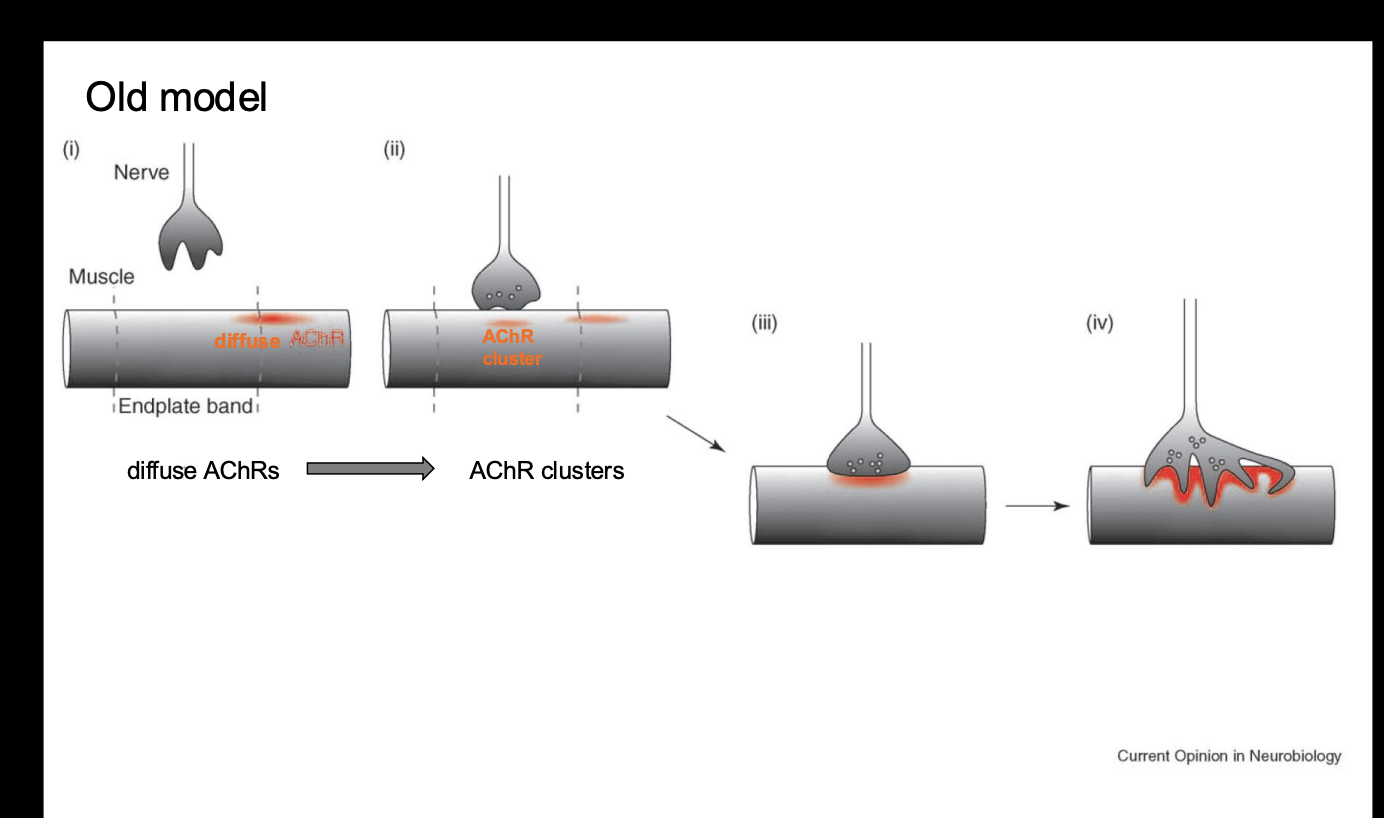
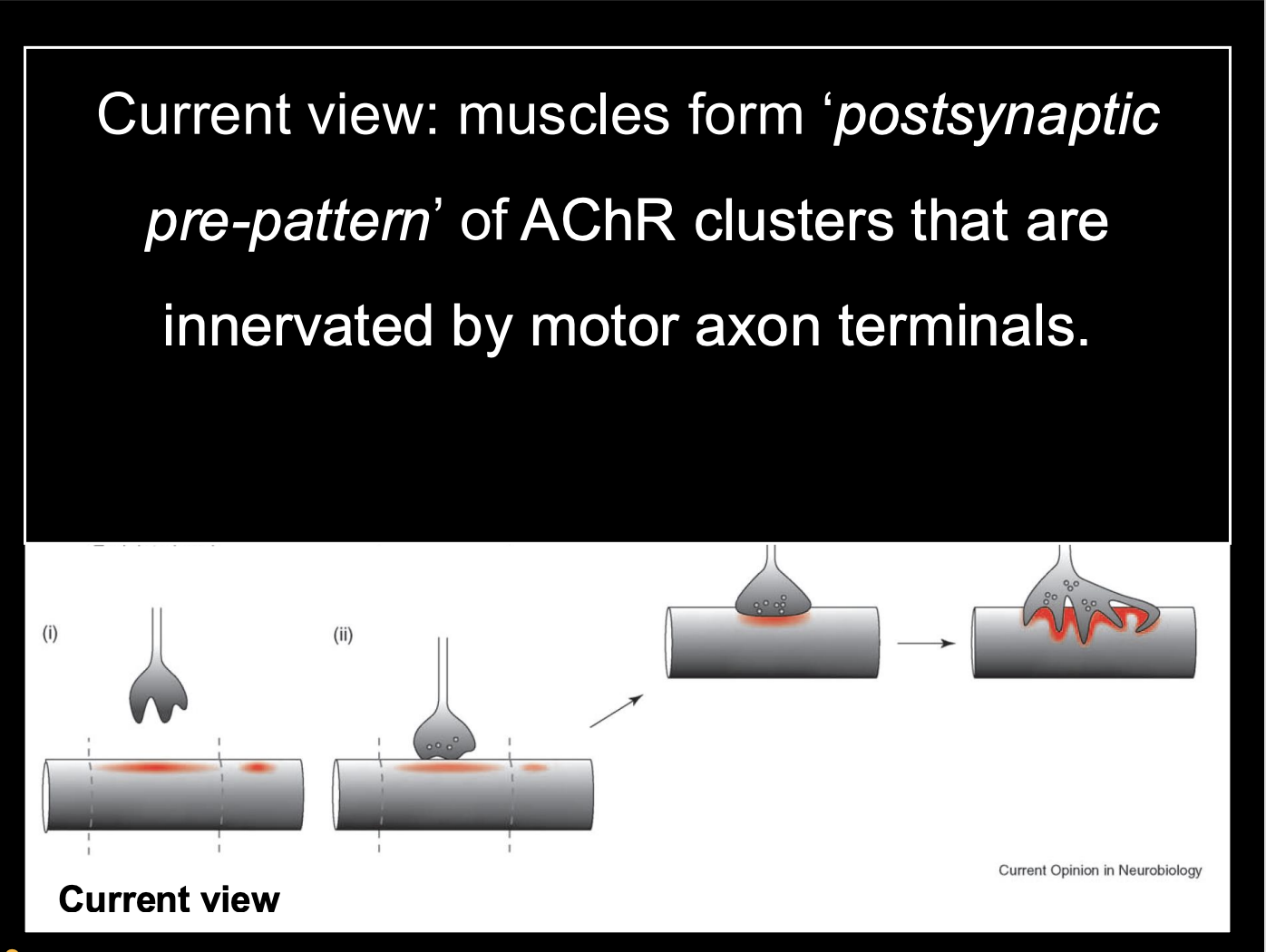
Overall: obervations showed the old ‘axoncentric model of synaptic formation
As motor growth cones arrive at the target muscle, AChRs aggregate at the site of innervation (presumptive synaptic site)
In addition→ transciption of AChR subunit mRNAs is confined to sub-synaptic muscle nuceli
All the while→ pre and postsynaptic cytoskeletal changes occur→ turning the growth cone from an exploratory organelle to a specilaised terminal
tranforms the postsynaptic site to an equally specialised region via
folding the postsynatpic membrnae
secretion of a basal lamina in the synaptic cleft
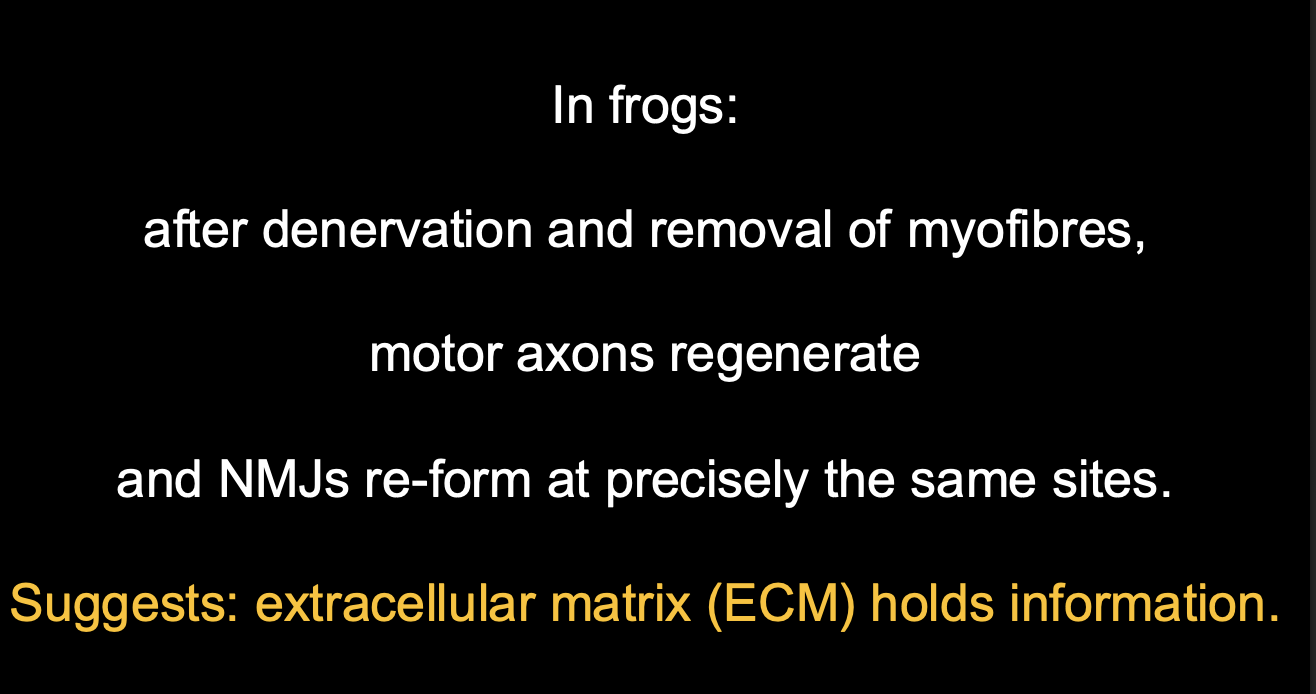
But where does the synaptogenic signal come from?
The basal lamina of NMJs
How was this found out?
McMahon and Sanes
denervated or eliminated the target muscle
Result:
regenerating NMJs reformed precisely as the original sites
only if the basal lamina was intact
THERFORE
synaptogenic signal must be from the basal lamina of NMJs
The extracellular matrix holds information
How was the synaptogenic activity biochemically purified
Procedure:
electric organ of torpedo marine ray (essentially a giant cholinergic NMJ) as basal lamina source
Assay for synaptogeneic activity for clustering
What was identified:
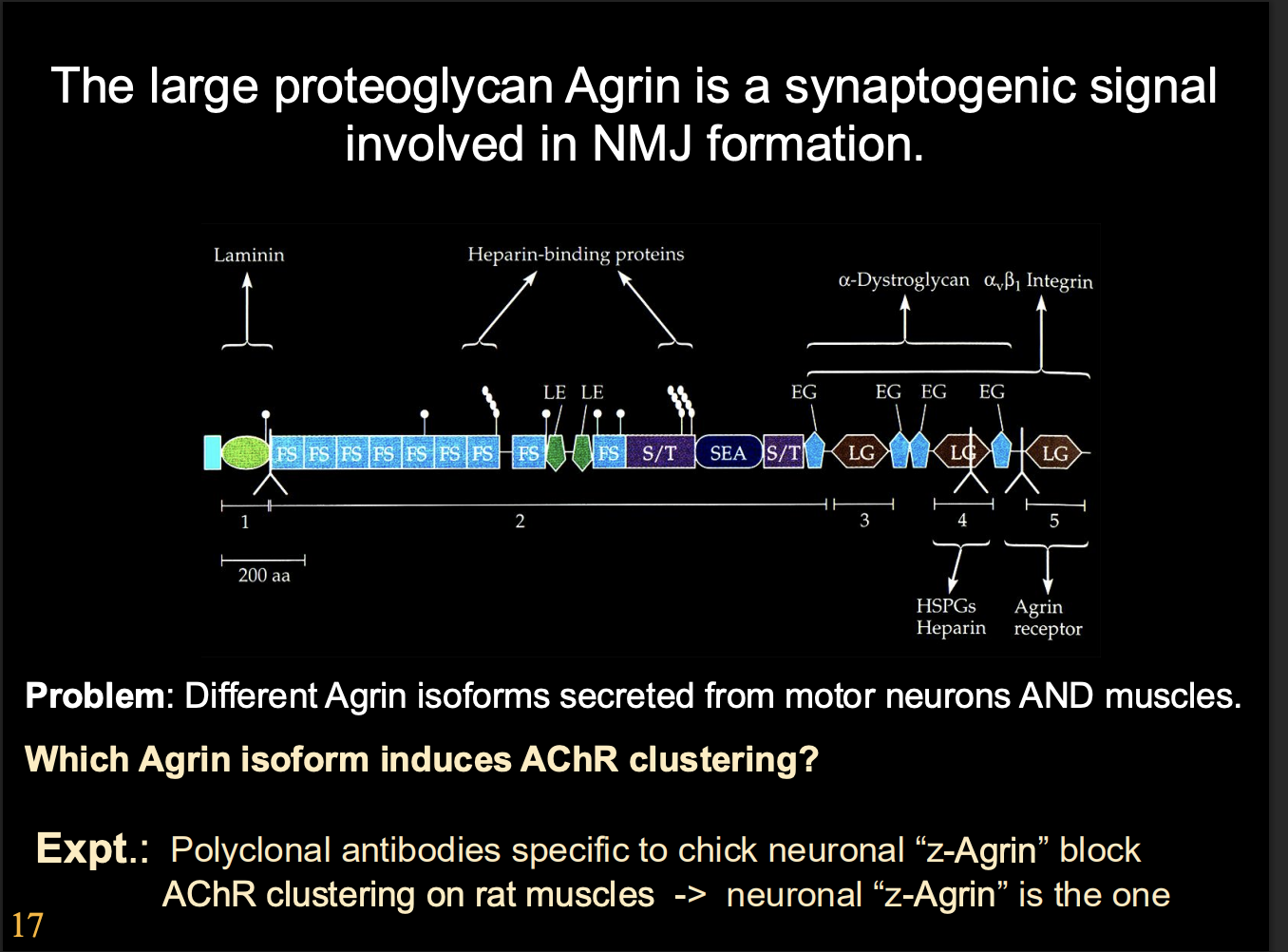
Large heparin sulphate proteoglycan→ Agrin
Has domains that interact with laminin
Other synpatogeneic signals from basal lamina
Where was Agrin found to be present
In motor axons as they made contact with their target muscles
Conformational evidence for agrin’s function
Knock out in mice
→ Severely impaired NMJ formation
But there are many types of agrins expressed in muscles and in the CNS, which one is used?
Alternively-spliced isoforms of agrin
How found each one for synaptic formation:
antibodies for different Agrin isomers
Results
z-Agrin were essential for synapse formation
So from this evidence, agrin is throught of as…
the key synaptogeneic signal
which acts of the growth cone
this was based on mutant phenotypes→ so not completely show the whole picture
Agrin receptor complex at the NMJ
Agrin receptor complex
with Muscle-Specific Kinase
Scaffold formation
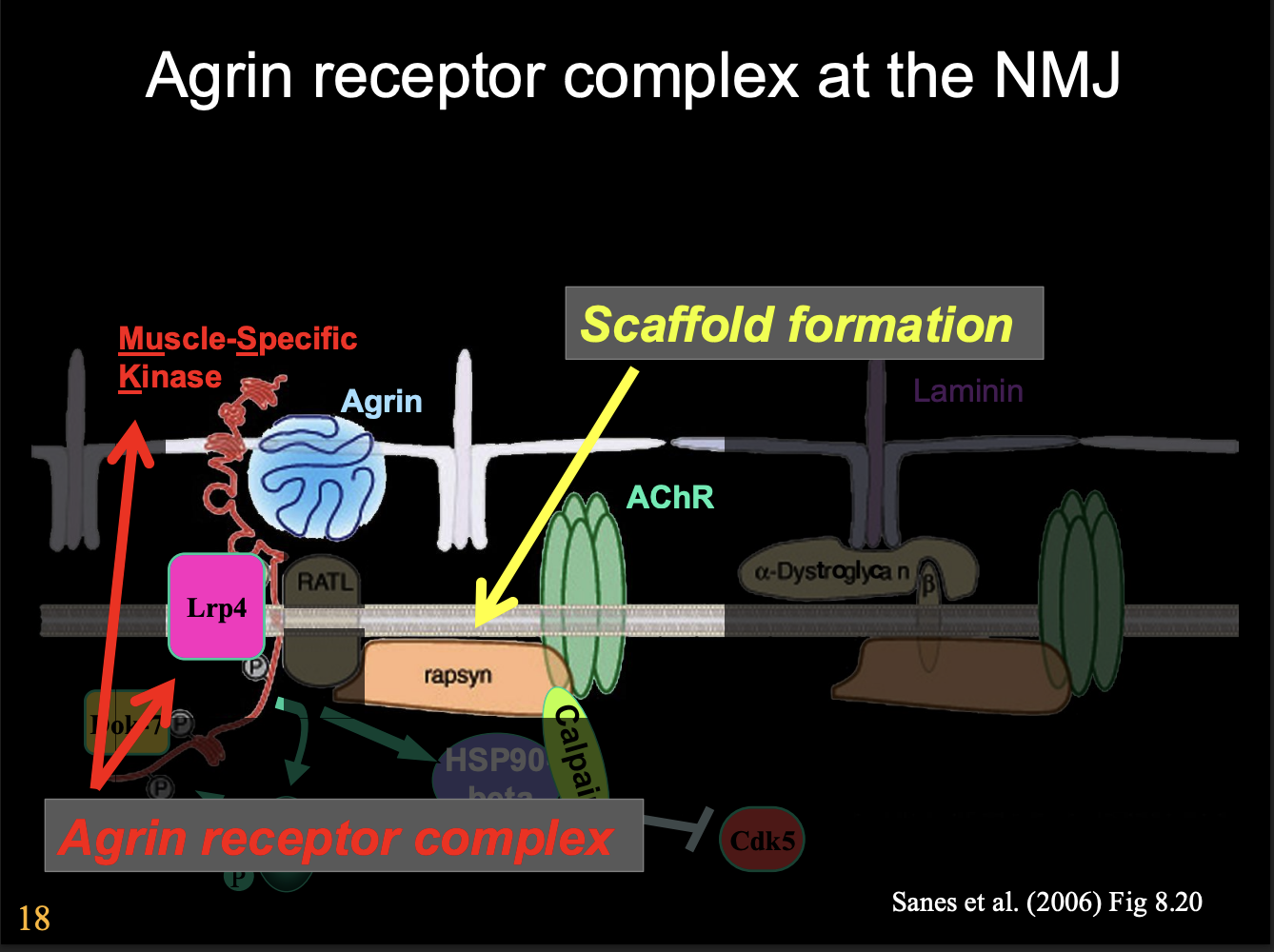
Overview of Agrin receptor complex mechanism
Agrin binds
Activates MuSK
activates Lrp4
activates HSP90
sequesters protease
allows cdk5→ bind and anchors down cytoskeleton
another kinase cascade
break up whole thing??
New idea of the role of Agrin due to other evidence
Agrin is a maintenance or ‘anti-dispersal’ factor
Stabilises neuromuscular junctions
by protecting subsynaptic AChR clusters from degradative processes that occur at extra-synaptic sites
What is the evidence for this
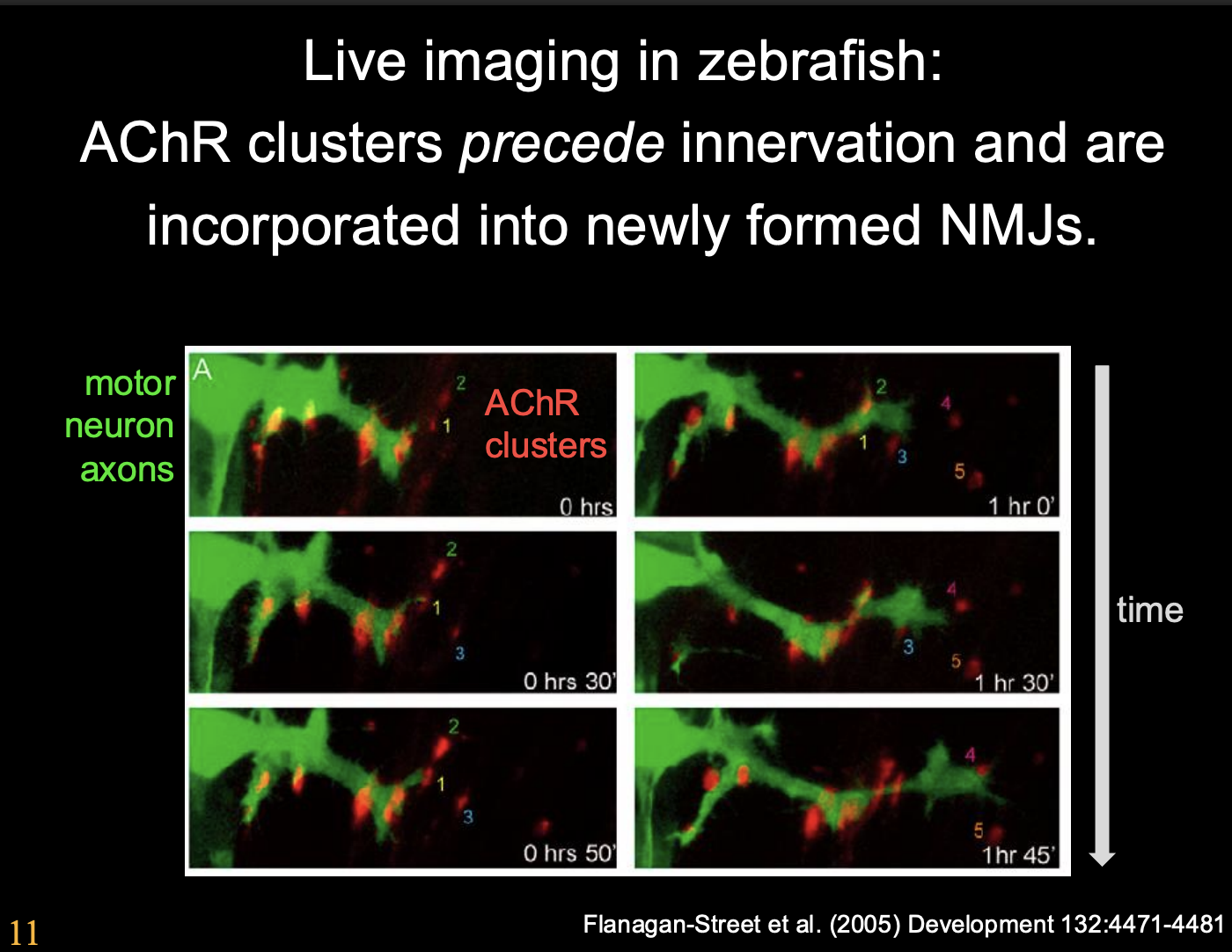
Zebra fish live imaging→ NMJ formation in zebrafish and of mucules in culture
Show: AChRs cluster at presumptive synaptic regions before and also at the absence of motoneuron innervation
Approaching motoneuron growth cones preferentially innervate these pre-existing AChR clusters
Animals mutant for agrin→
AChR clusters also form in the absence of Agrin
AChR clusters require Agrin to be maintained→ following motor axon innervation:
suggests: motoneurons secrete some AChR cluster dispersal agent (now known to be ACh) which helps to maintain them
More info on the Zebra fish live imaging evidence
Procedure:
Label with homeobox gene→ labelled the motor neurons with GFR
Label AChR receptor in red
note: not all labelled to ensure some are still functioing
Observe overtime how the AChRs cluster with growth cone approach
Observations:
growth cones contact receptors and get larger and increase branches to clusters
post synaptic muscle is guiding the growth cone→ not a passive entitiy
Conclusion:
AChR clusters precede innervation and are incorporated into newly formed NMJs
The post synaptic muscle has some pre-patterning of clusters
More information→ Evidence that AChR clusters require Agrin to be maintained
Procedure:
Agrin mutant mouse
Remove ACh synthesis
Results:
AChR clusters can form and remain
Why: because there is no acetly choline to signal AChR cluster dispersal
Conclusion:
ACh is used as an AChR cluster dispersal factor
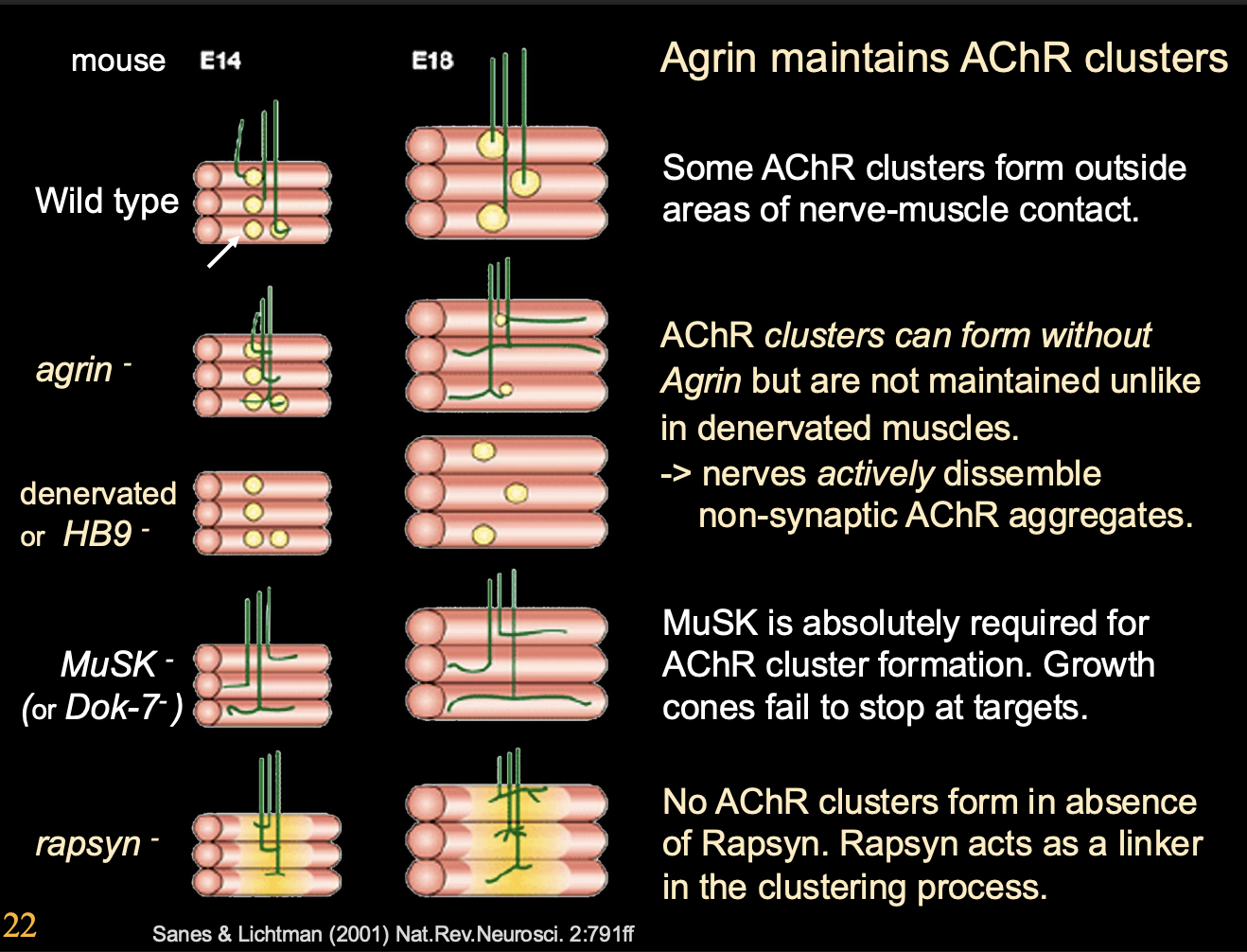
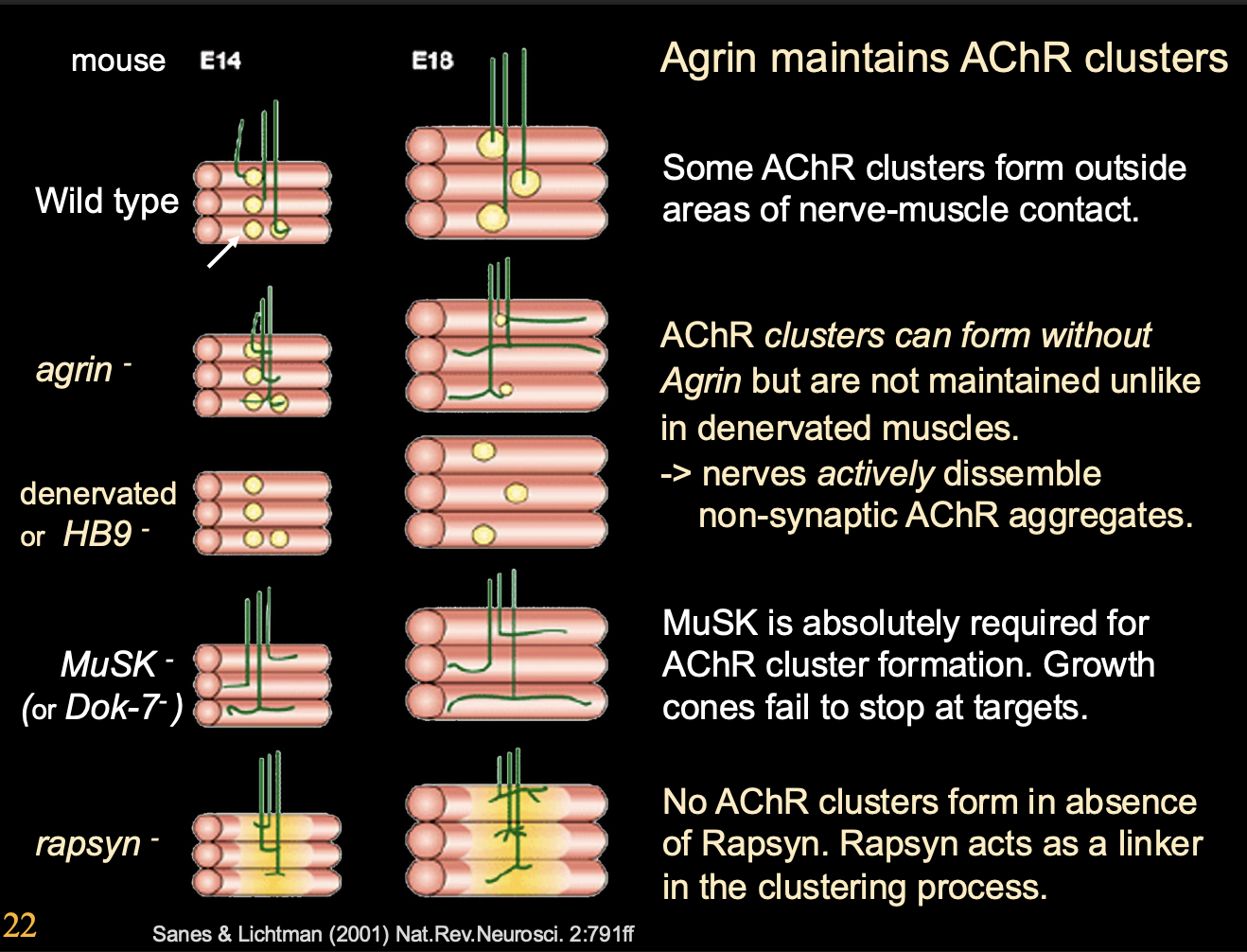
More in depth experimental evidence about how Agrin maintains ACR clusters
Procedure
Mice with various mutants
no agrin
denervated
double mutant
Result:
No agrin→ AChR clusters can stil form BUT not maintained
Denervated→ Clusters are mainted but not innervation
Double mutant→ ACh disperses extra-synaptic clusters→ NOT mainatined by nerve derived Agrin
Two conclusions from this
Nerves actively dissemble non-symaptic AChR aggregates
Simulatenous removal of Agrin and ACh
OVERALL: Agrin maintains the clusters
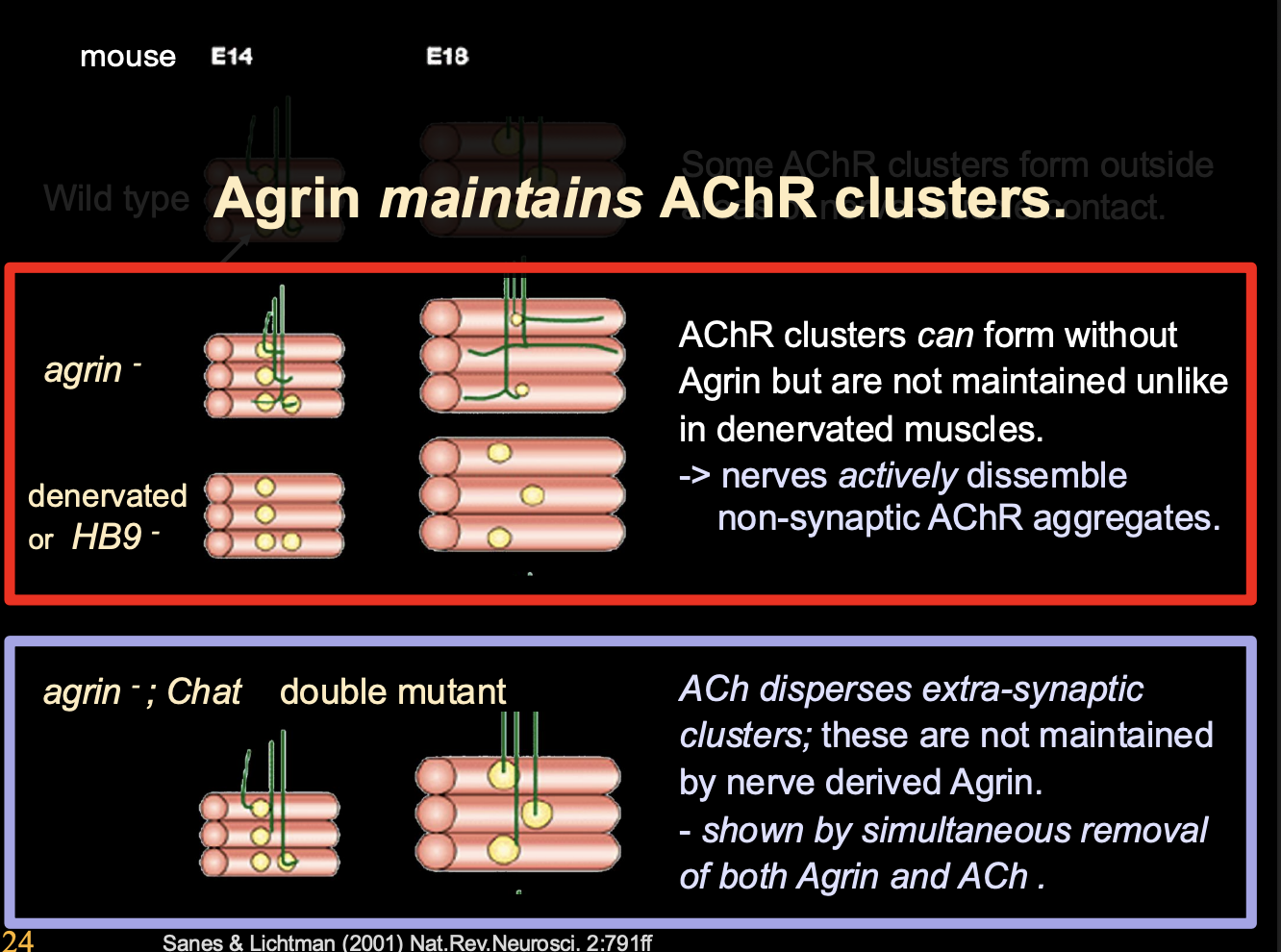
Therefore this experiment lead to the current view→
Postsynaptic pre-pattern→ AChRs clustered before the growth cone arrives
ACh from nerve terminal can diffuse beyond the immediate synapse region and lead to dispersal of extra-synaptic AChR clusters
Agrin is both an activator of agrin recetptor complex and ‘Anti-dispersal agent’ (maintainence)
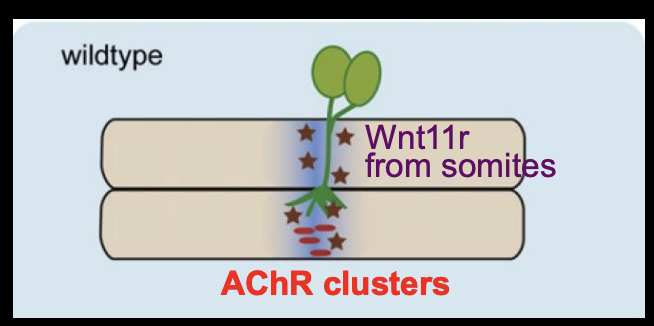
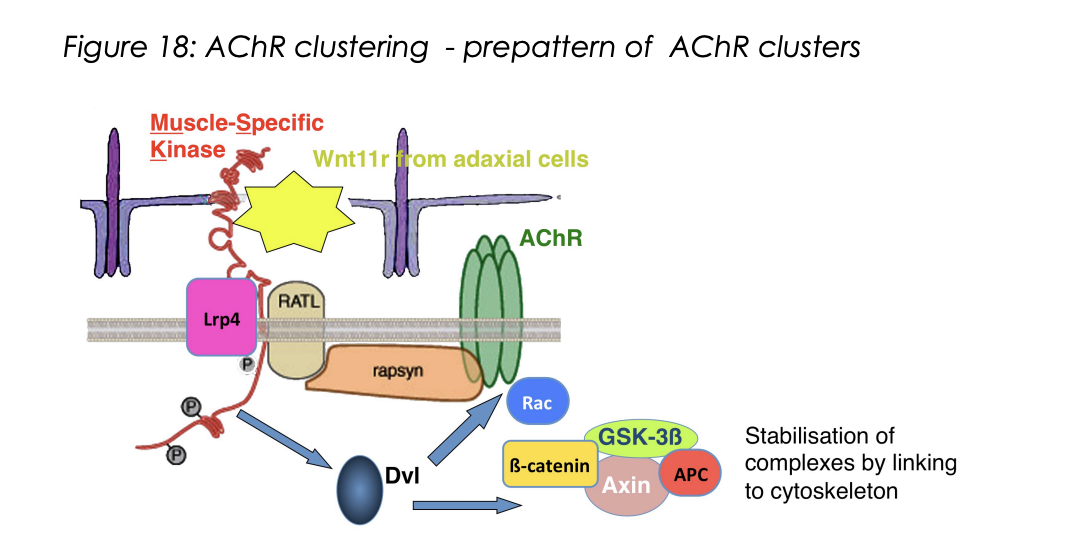
How is the muscle pre-patterned? (remains controversial)→ Zebrafish evidence
Zebra fish
Wnt11r acts as a ligand and activates MuSK on muscles
wnt11r from dorso-lateral somites
Induces AChR clusters in central muscle region
Also guids motor axons along this central muscle region
by forming a corridor that is attractive to the growth cones
overall: Wnt11r works as a third party mattch maker for the patterns to match the growth cone approach.→ like how a timetable brings the lectuere and student together without the communication between the two
How is the muscle pre-patterned? (remains controversial)→ Mouse evidence
Mouse model→ Wnt-MuSK signalling
MuSK kinase being inherent active at low level
therefore→ oldest part of the muscle ends up with the greatest MuSK activity
therefore→ AChR clustering
Agrin then induces further what
Clustering
stabilisation
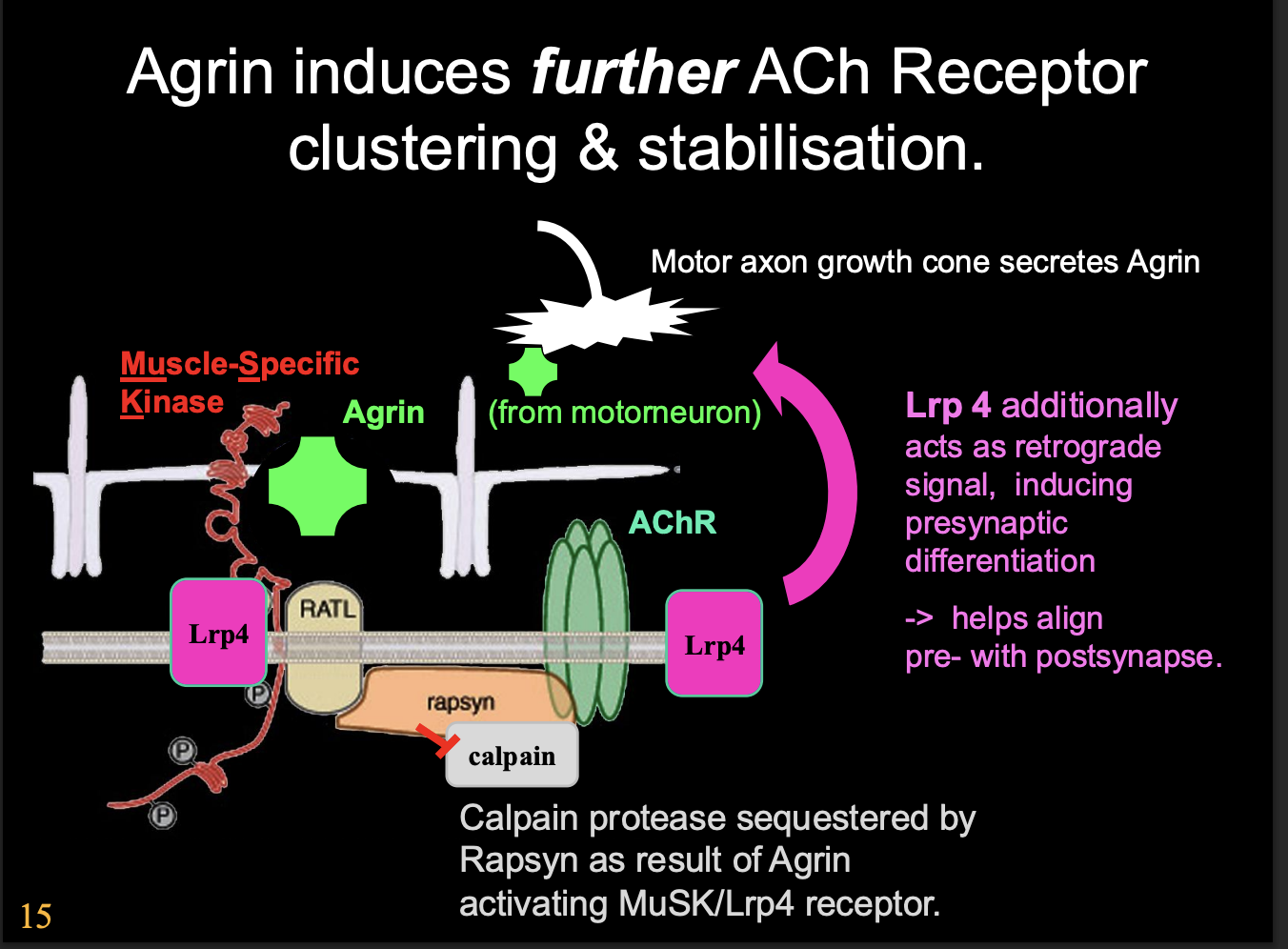
How does it do this
Agrin is large and when depoisted on motor axon terminal→ will remain localised in the ECM as the synpase ONLY
Activaes Agrin receptor complex
Activates on MuSK
Activates Lrp4
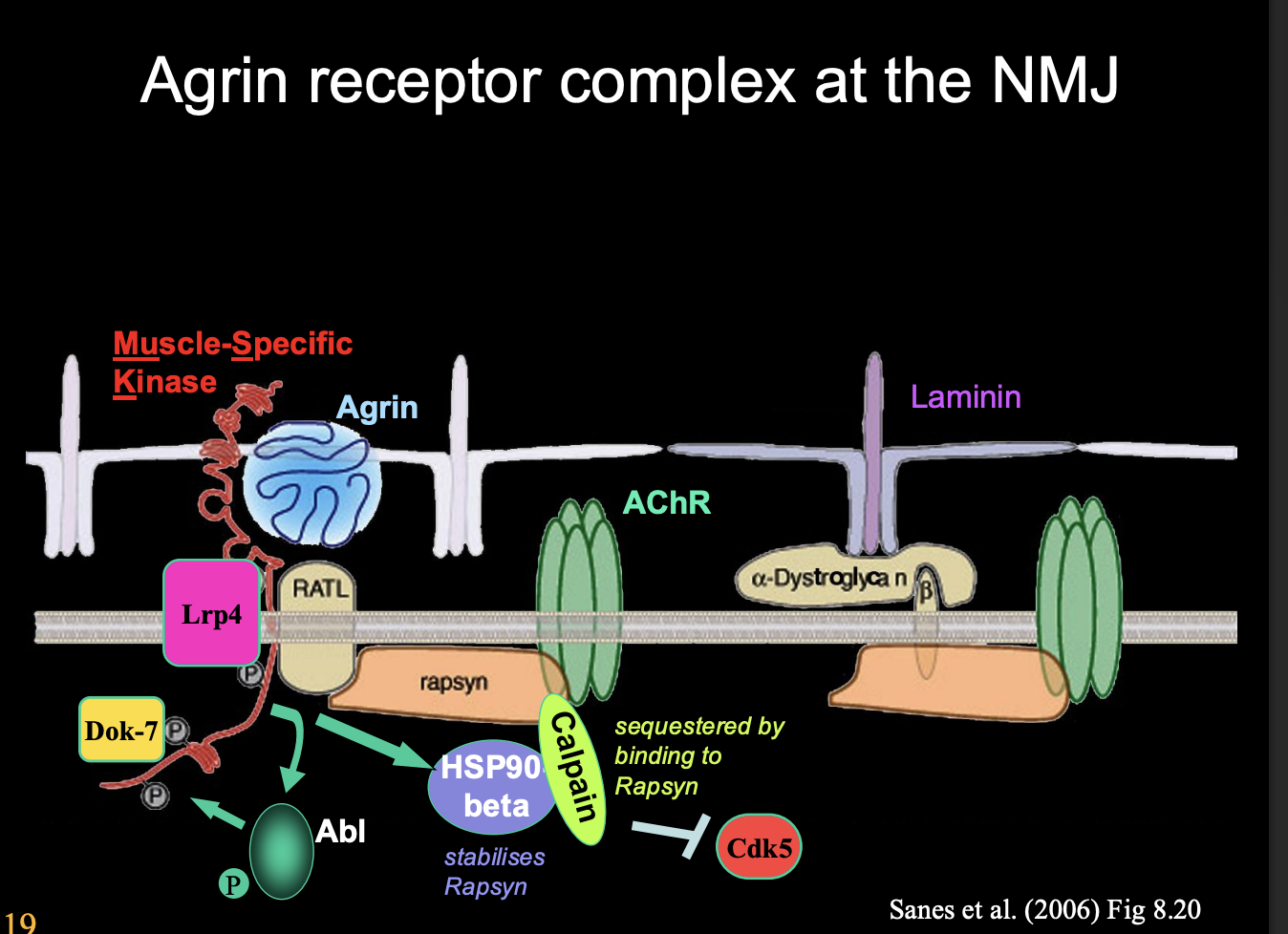
Calpain protease is sequested by Rapsyn (a scaffolding protein)
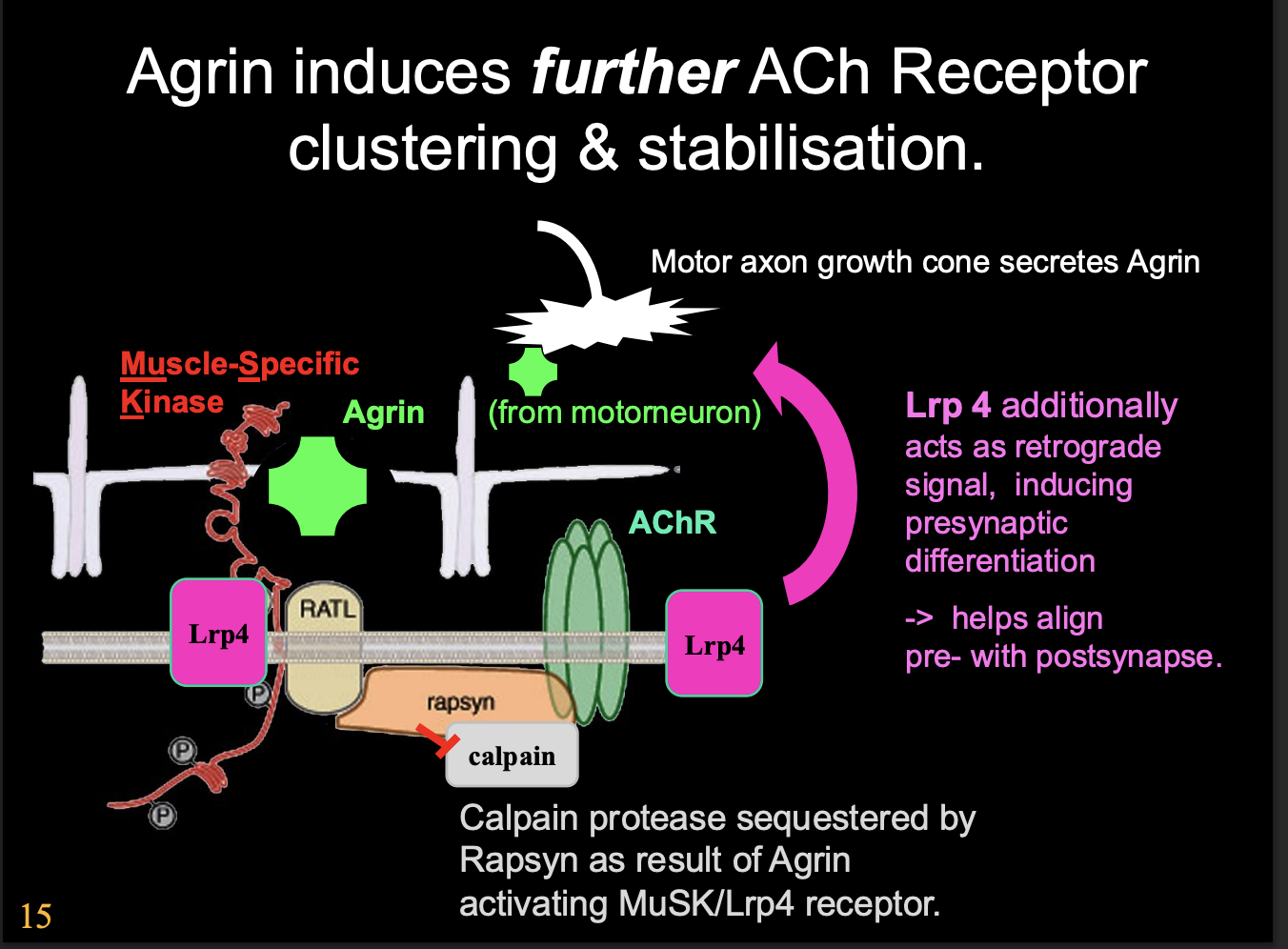
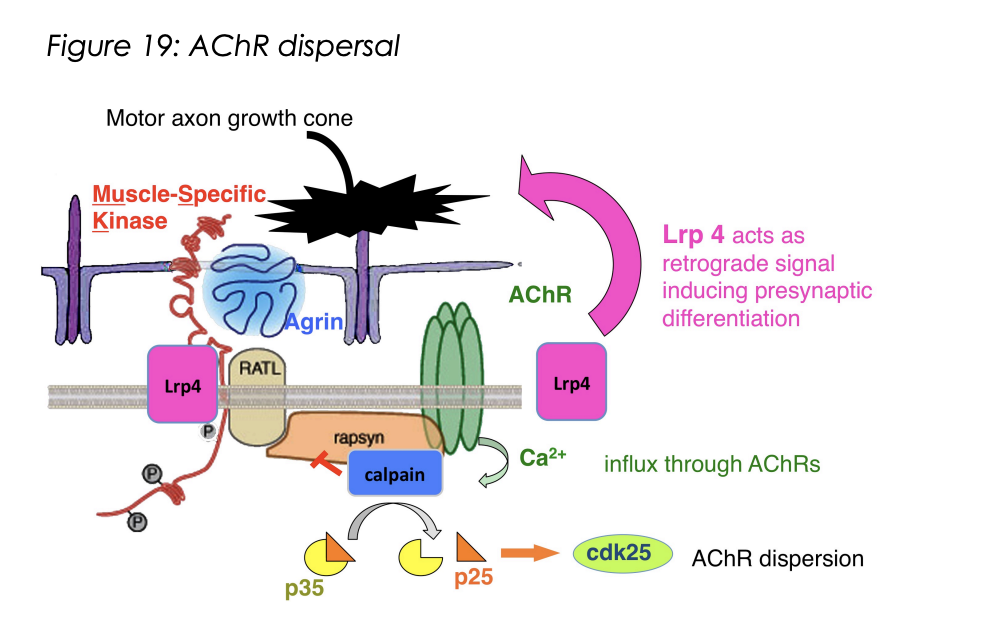
What does the activation of Lrp4 also do
Lrp4 also acts as retrograde signal→ inducing presynaptic differentiation
helps to align pre with postsynapse
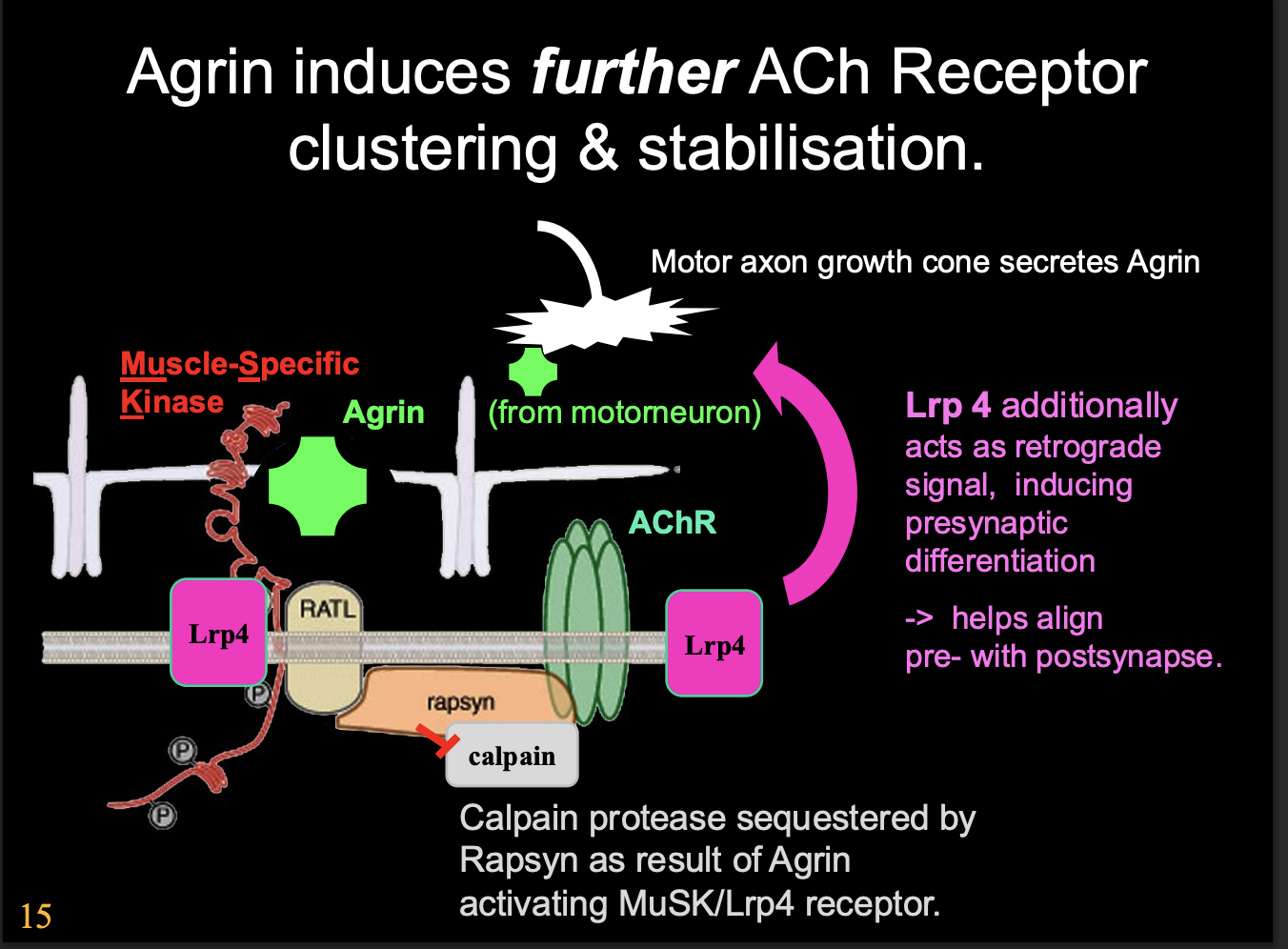
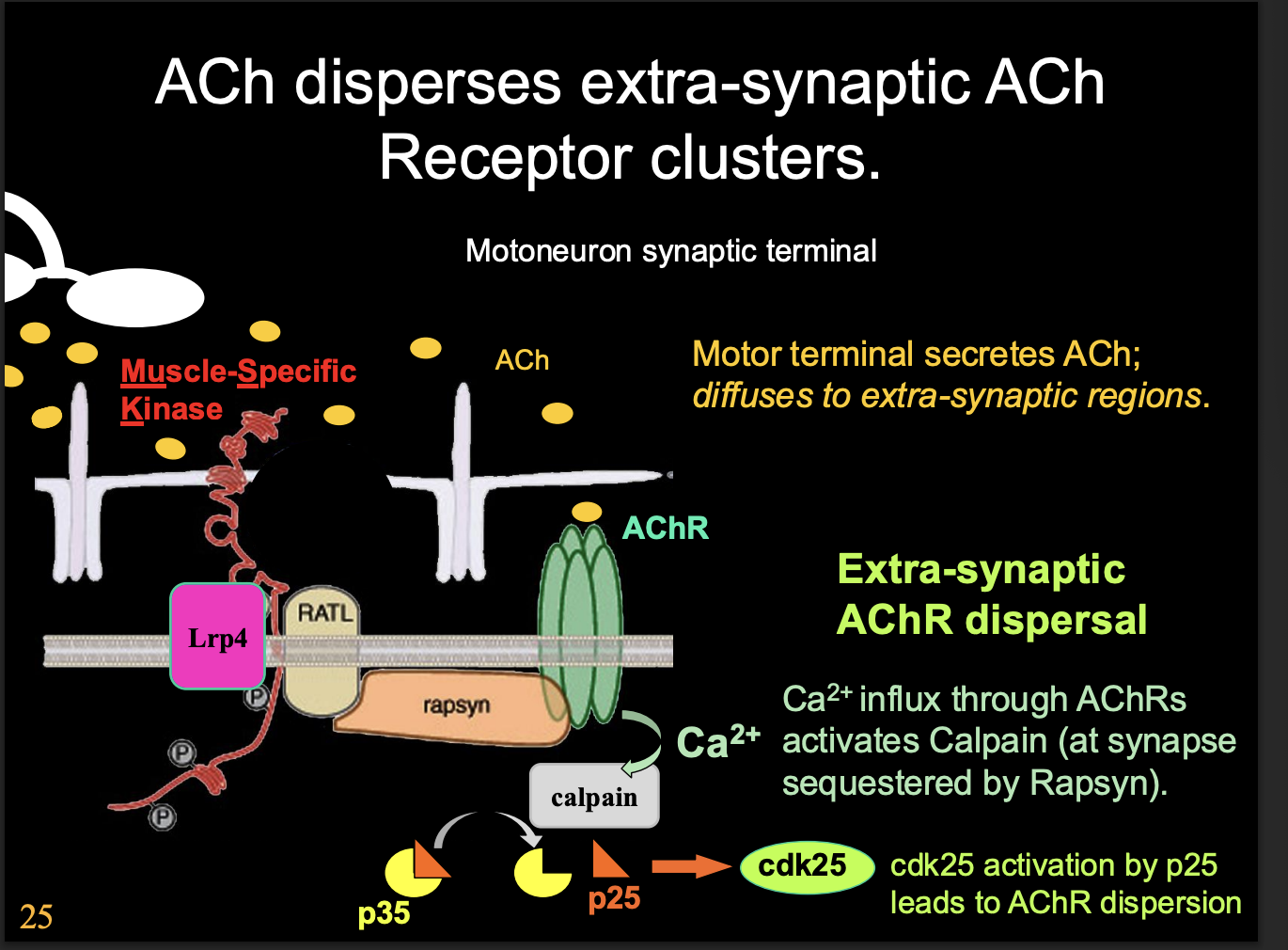
How does ACh release casue AChR dispersal
note: ACh is normally blocked by large agrin
Dispersal is triggered by AChR mediated clacium influx
Activates Calpain protease ( which is normally inactivated by sequestration due to agrin receptor complex activation)
activates kinase cascade
cdk25 activation by p25
promotes dispersal and internalisation of extra-synaptic AChRs
Why is AChR dispersal needed?
to get rid of extra-synaptic AChR
ensure that the synapse is precisely aligned
How does Agrin work to maintain pre-esxisting AChR clusters (seen above too)
Locally→ antagonising the ACh dispersal effect
Globally?→ No
extra-synaptic receptor clusters will still be affected by acetyl choline
THEREFORE: this allows for synapse refinement by elination of extra-synaptic sites
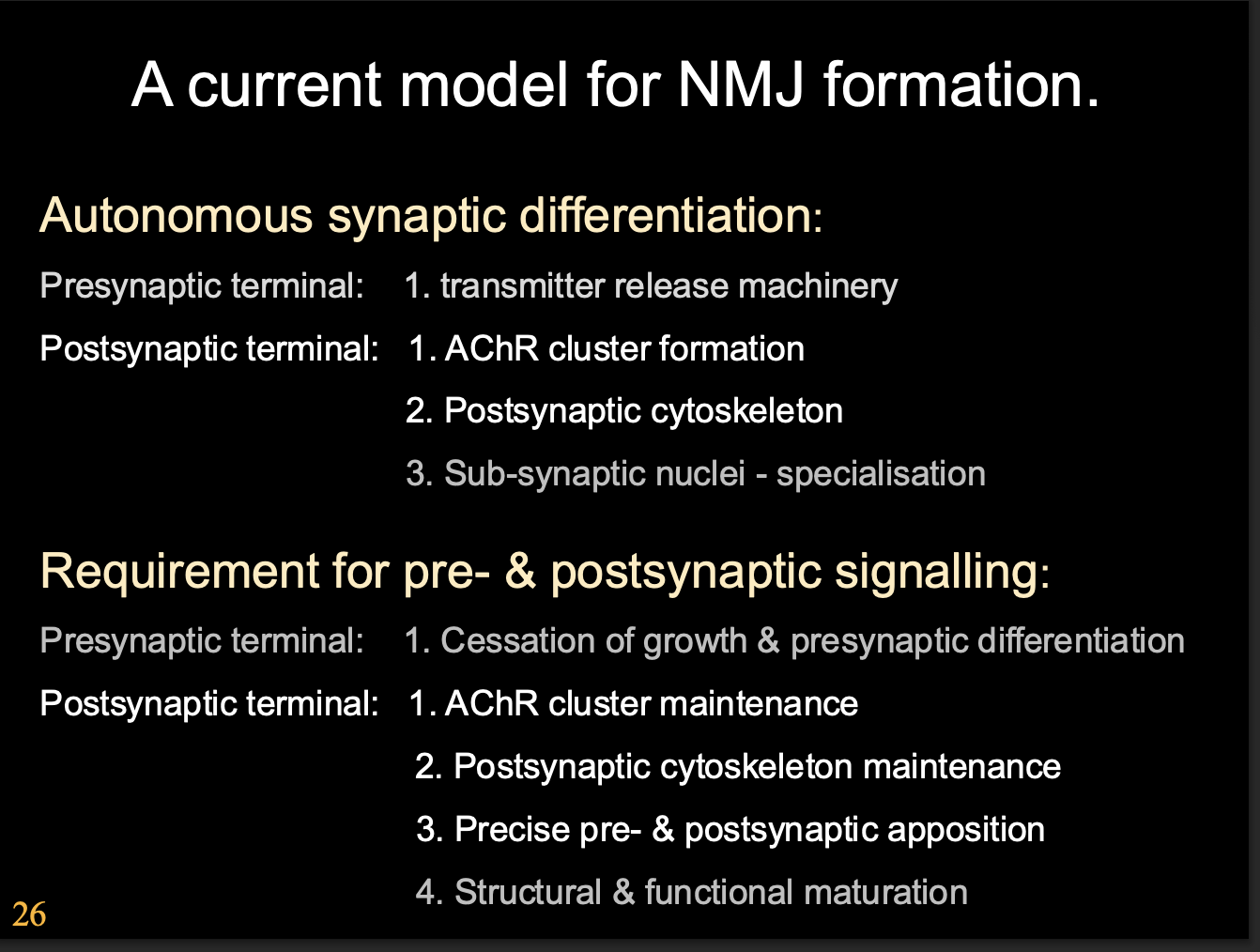
The current model for NMJ formation
Autonomous synaptic differentiation
Presynaptic terminal→ Transmitter release machinery
Postsynamptic terminal→
AChR cluster formation
Postsynaptic cytoskeleton
Sub-synaptic nuclei- specialisation
Requirement for pre and postsynaptic signalling
Presynaptic terminal→ Cessation of growth and presynaptic differentiation
Postsynaptic terminal→
AChR Cluster maintenance
Postsynaptic cytoskeleton maintenance
Precise pre and post synaptic apposition
Structural and functional maturation
The next few cards are on more details of the parts of the Agrin pathway: Downstream of Agrin what happens First stage of synaptic formation
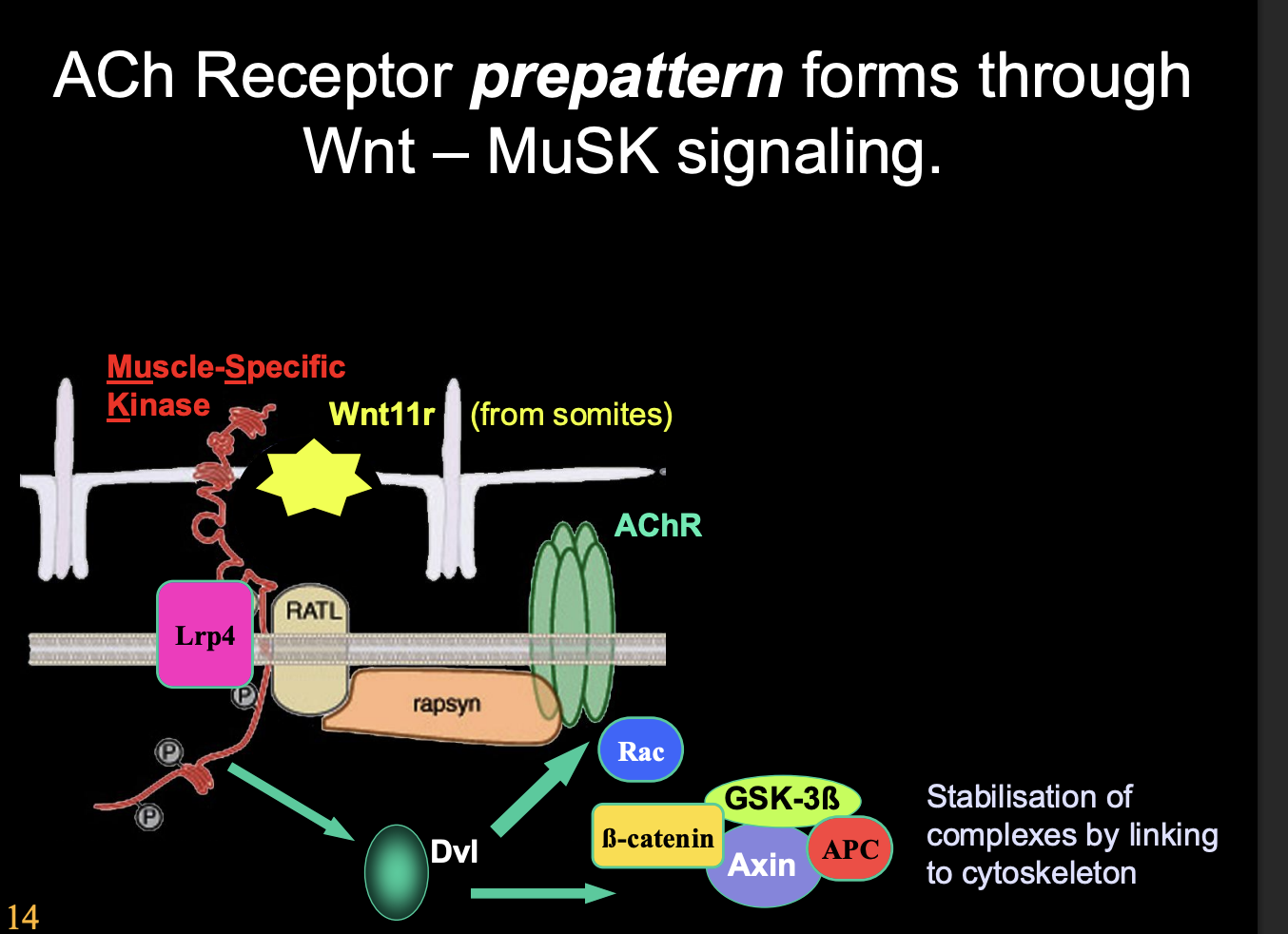
Lpr4→ low density lipoprotein receptor-related protein
Associates with and signals through a Muscle-specific receptor tyrosine kinase (MuSK)
MuSK also binds Wnts
In zebra fish→ myotime secreted wnt11r activats MuSK signalling in the central domain of muscles
triggers formation of AChR clusters
At the same time→ Wnt11r interacts with MuSK present on the grwoth cones of motorneurons
Guides them along this central corridor→ towards the pre-formed AChR complexes
Remains to be shown if Wnt-MuSK signalling also peforms such roles in mouse
Second stage→ What happens once motorneuron growth cones have reached muscles…
Agrin comes into play
Binds to receptor complex
MuSK phosphoorylates itself
Triggers an intracellular signalling cascade
Leads to assembly of te postsynpatic apparatus
thrid stage→ One structrual component is Rapsyn
Postsynapse protein
acts as a scaffold
mediate interactions between MuSK, AChRs and other postsynaptic cytoskeletal elements
What does the lack of presynaptic specilaisations (e.g MuSK or agrin-deficient mice) suggests
Presynaptic differentiation requires a retrograde signal from the muscle
What does this retrograde signalling
Lpr-4 of the Agrin receptor complex
signals retrogradely to induce differentiation of the presynaptic motor axon terminal
Signals from basal synaptic lamina→ also induce presynatic specialisations (in regenerating motor axons)
e.g basal lamina-associdated Laminin-b2
How is refinement achieved
Dispersal and removal of AChRs from extra-synaptic regions
ACh released by motor terminals
diffuses beyong regions of synaptically laid down Agrin
As no argin→ AChR is exposed to ACh
mediate calcium influx
activates protease calpain
Activates cdk25
Auntonmous vs non-autonomous features of synapse formation
There is alot of intrinsic signalling between pre and post synaptic partners
But→ some of these happen autonomously and some require interactions between partner cells
non-cell autonomous
Autonomous processes
Muscles→ postsynaptic differentiation in absence of presynaptic motorneurons
Presynaptic differentiation→ growth cones appear inherently capabale of releasing neurotransmitter and presynaptic sites form in the absence of poastsynaptic targets
Made really wuickly but still really complex→ how is this?
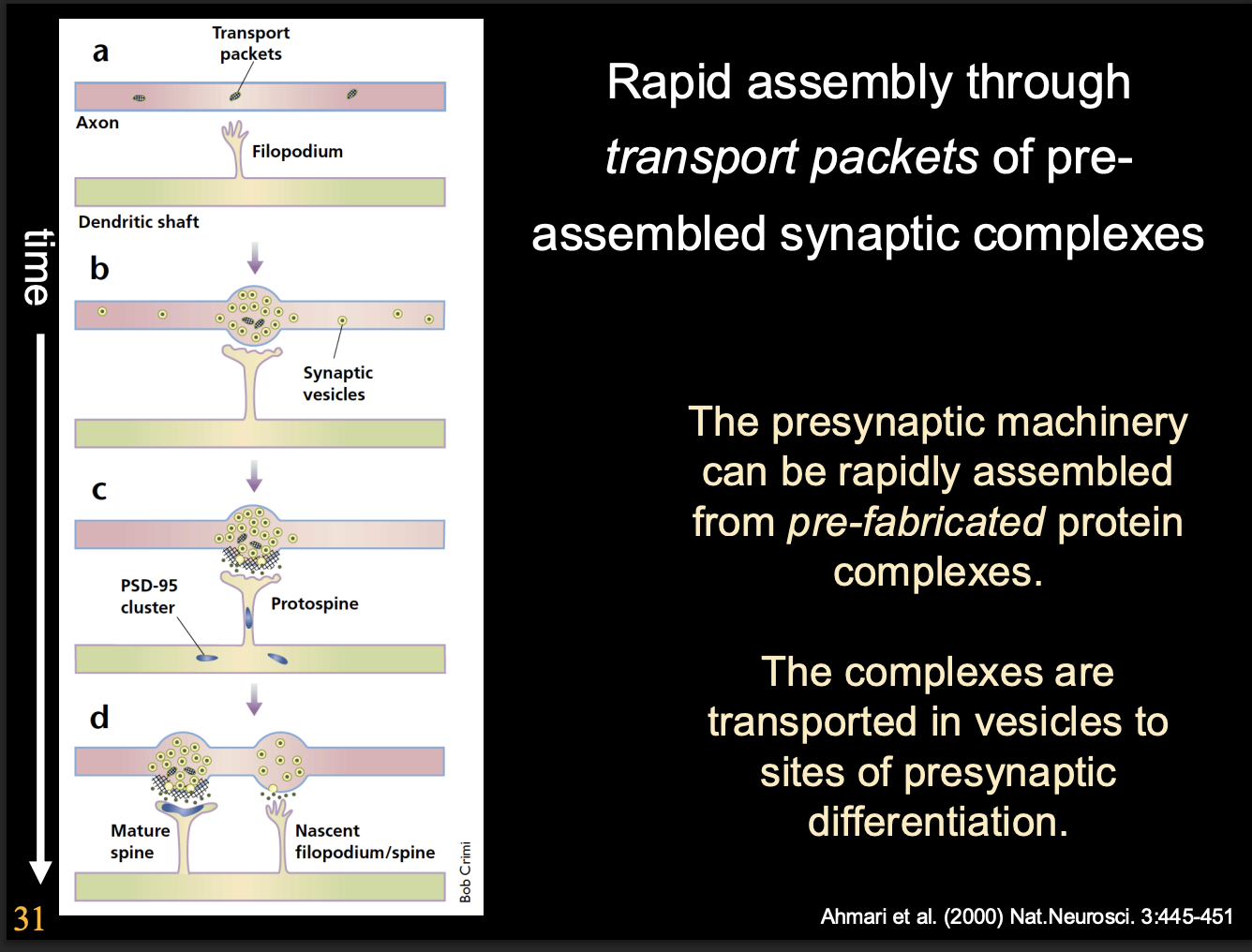
How has it been shwon the presynaptic differentiation is autonomous
Presynaptic apparatus is assembled from prefabricated modules
delivered by specialised vesicles
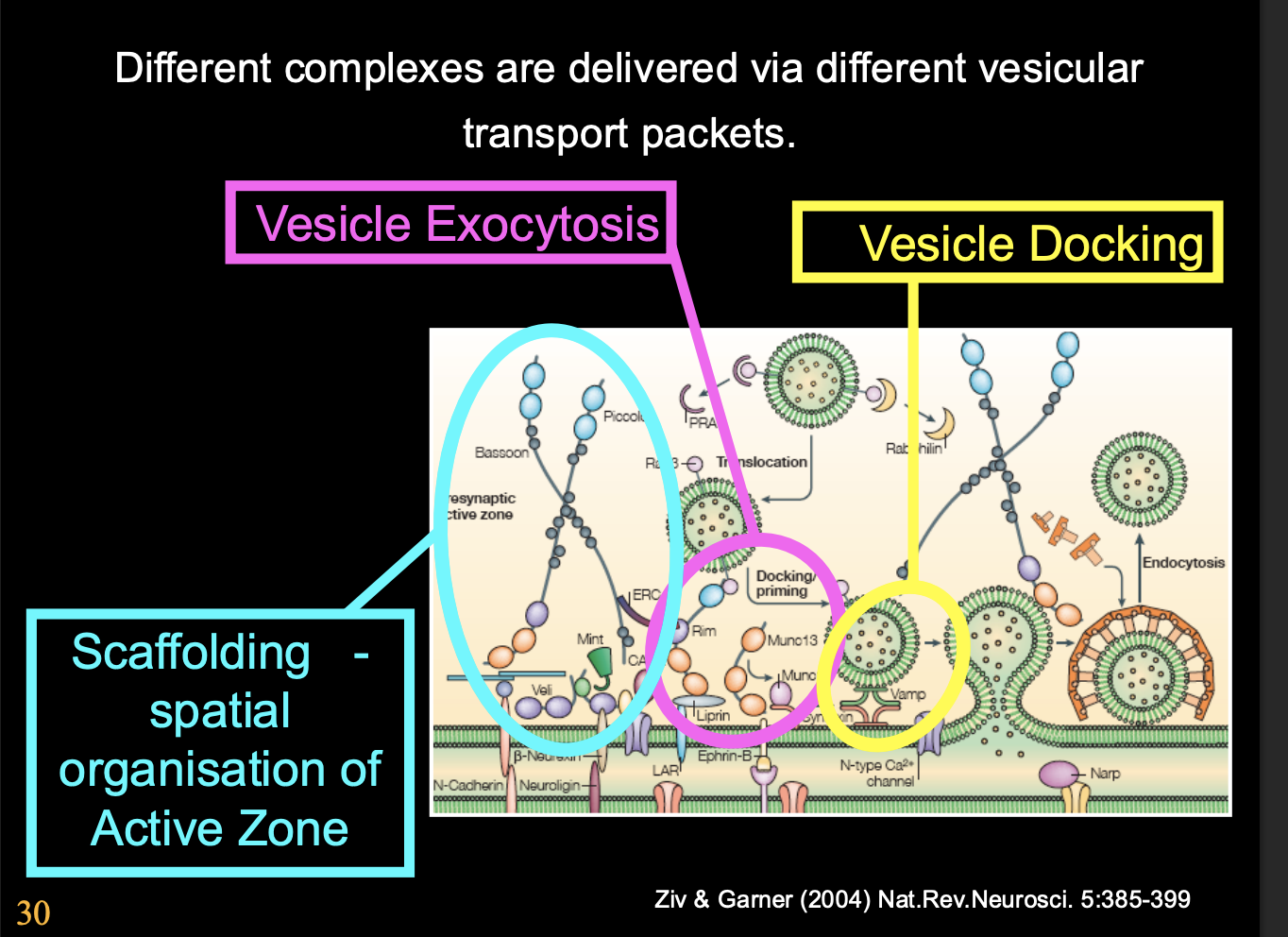
Why is it useful to use prefabicated modules
facilitates rapid formation of functional release sites
as soon as presynaptic cell comes into contact with postsynaptic cell
Are post-synaptic specialisations made from pre-fabircated complexes?
Unclear whether it is pre fabicated or assemble gradually
How do postsynaptic protein complexes assemble in the CNS
assemble on scaffolding proteins
containing PDZ domains
different proteins are responsible for clustering of different neurotransmitter receptors
Example of protein with PDZ domain
PSD-95 protein
prominent at glutamatergic synapses in vertebrates
Exmaple of specified scaffolding proteins NMJ vs CNS
NMJ
Rapsyn
CNS
Gephyrin
important for glycine and Stargazin for glutamate receptor clustering
So THEREFORE what is autonomous vs non-autonomous
Autonomous
Pre and post synaptic specialisation
Non-autonomous
Location
Alignment
Asjustmnet during development
Synapse formation in the CNS→ What happens to the growth cone as synase forms
changes from highly motile navigational organelle→ presynaptic structure
But what are the signals that induce these changes?
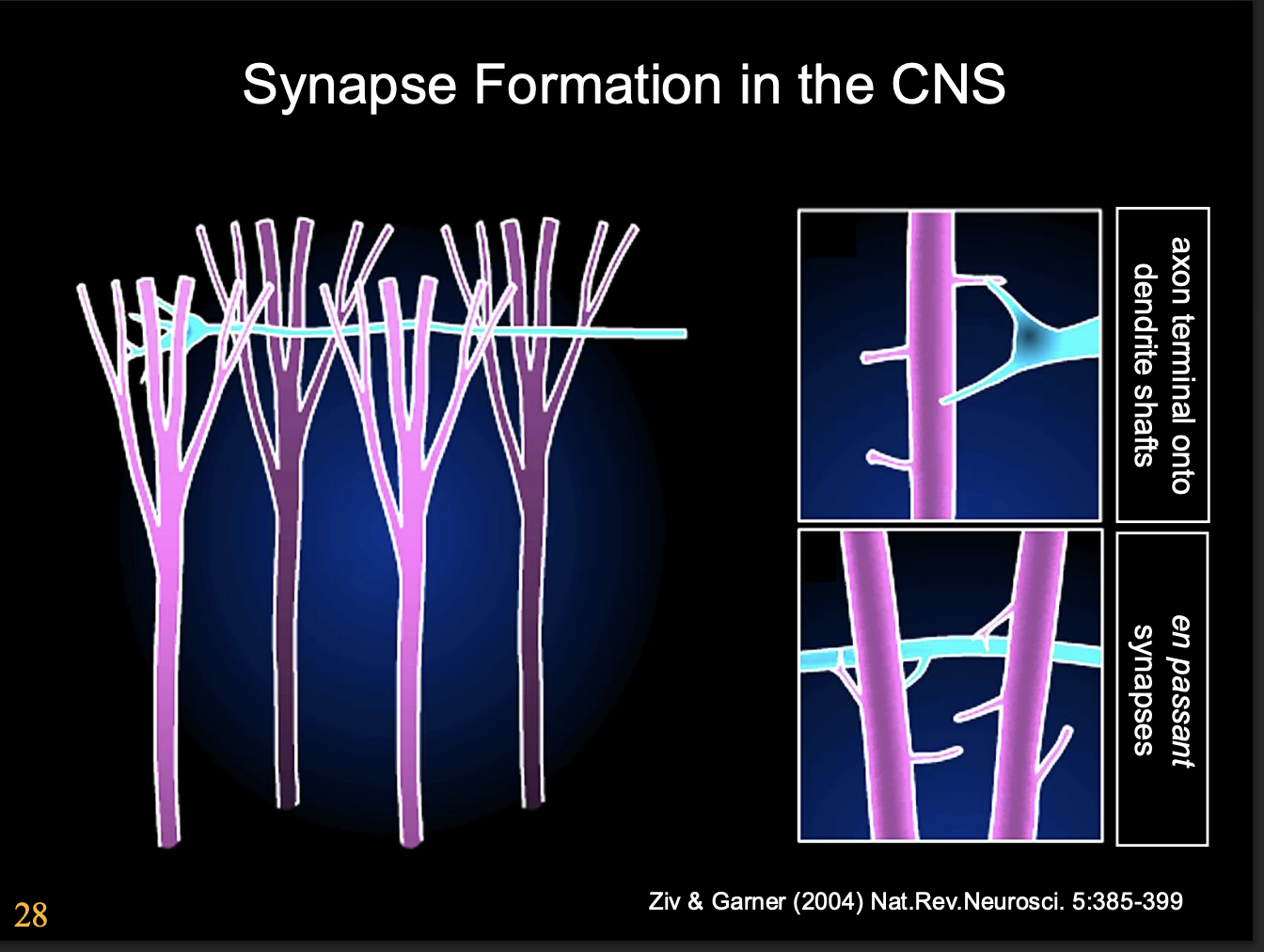
Why is the cerebellum a very successful model system
regular organisation
well defined development and connectivity
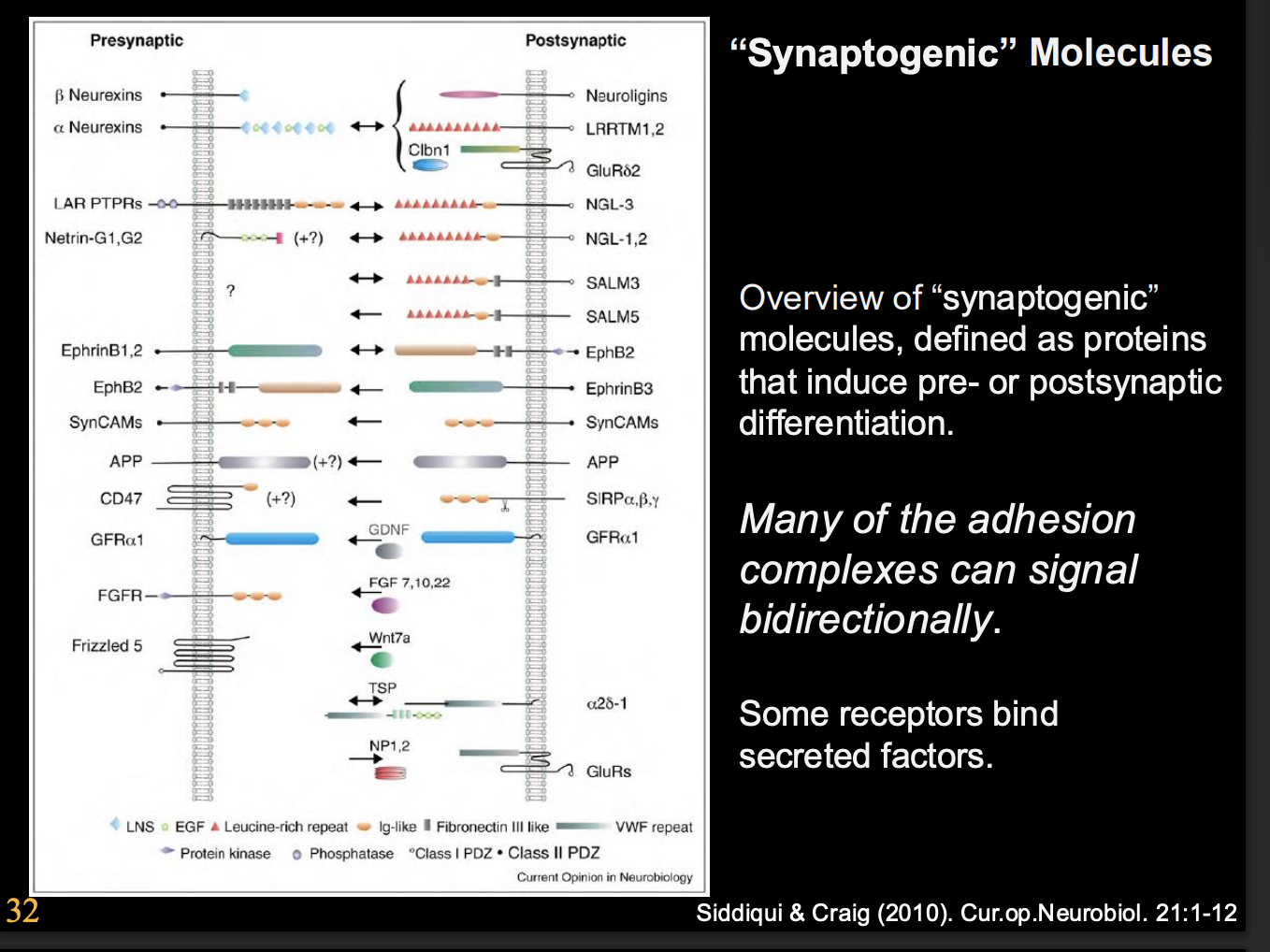
What are synaptogenic molecules
Proteins that induce pre or postsynaptic differentiation
many of the adhesion complexes can signal bidirectionally
some receptors bind secreted factors
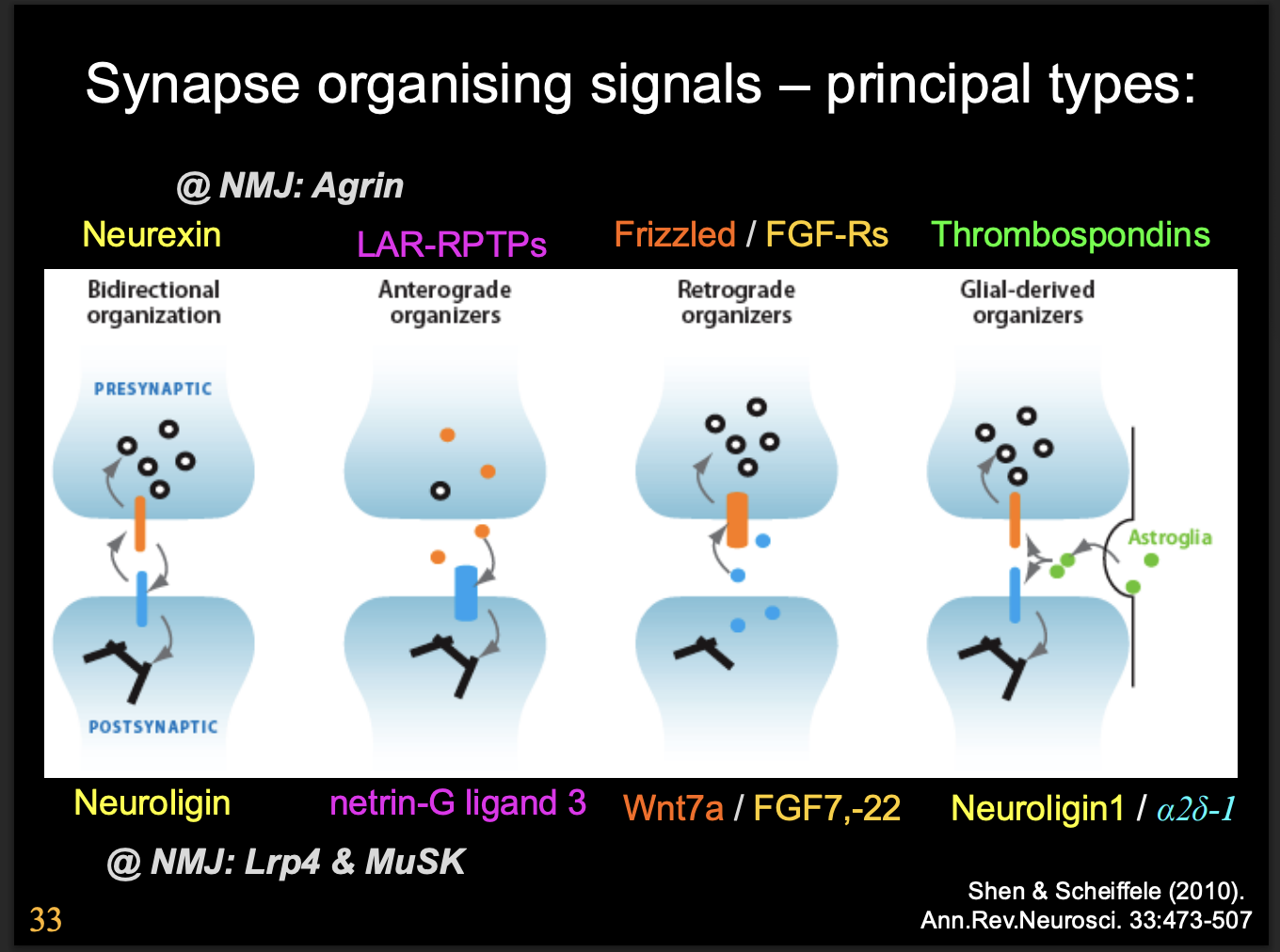
Principal types of synapse organising signals
Bidirectional organisation
Anterograde organizers
Retrograde organizers
Glial-derived organizers
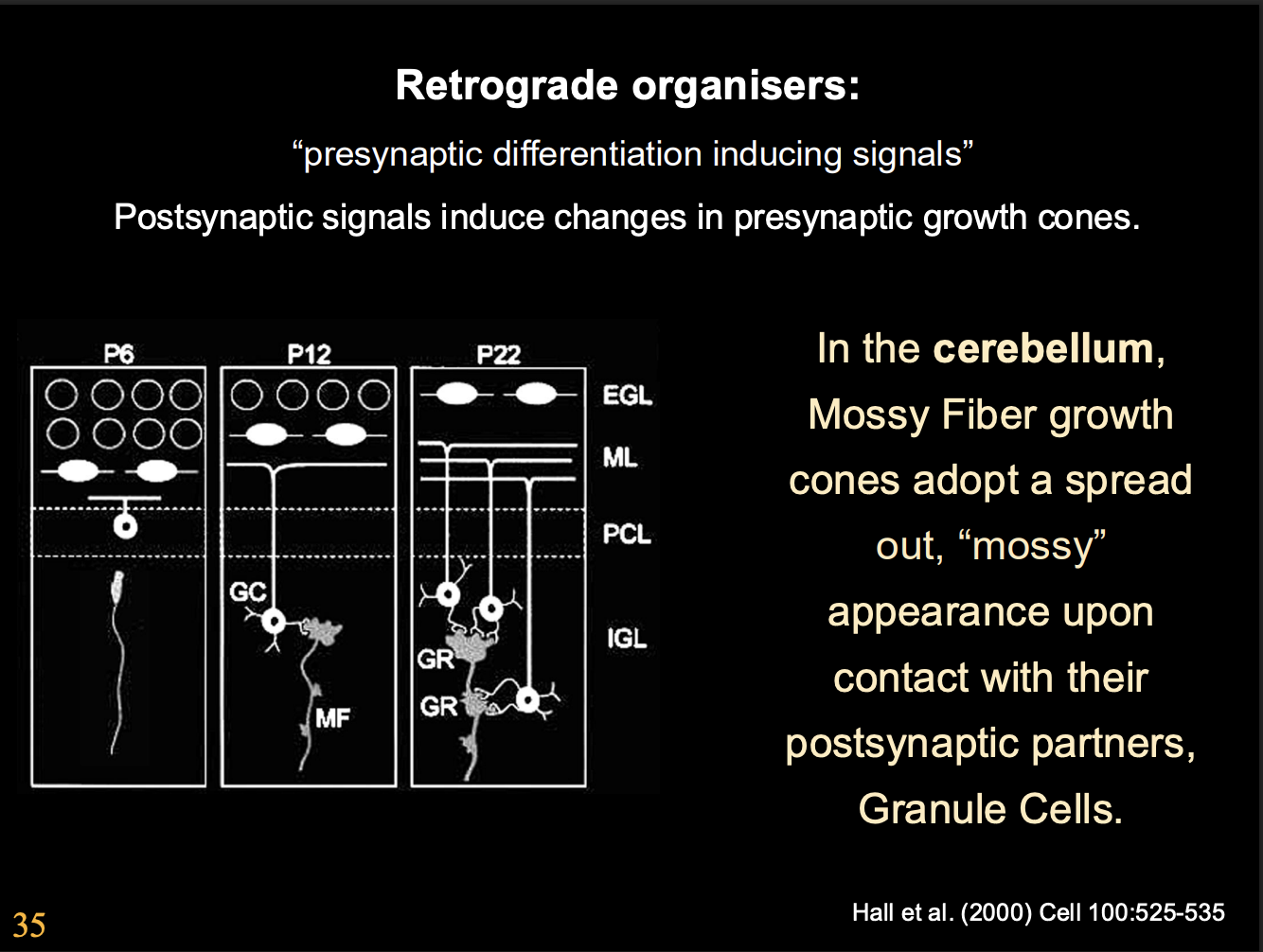
Retrograde organizers found in cerebellum
E.g Frizzled or FGFRs
secreted by postsynaptic cells (granule cells)
promote differentiation of presynaptic (mossy fibre) growth cones
Examples of these retrograde signals and what they specialise in
Wnt7A→ but not strictly required as Wnt7a mutant mice still get cerebellar synapses
but with a dealy
THIS SHOWS→ signals are complex and there are often many of them
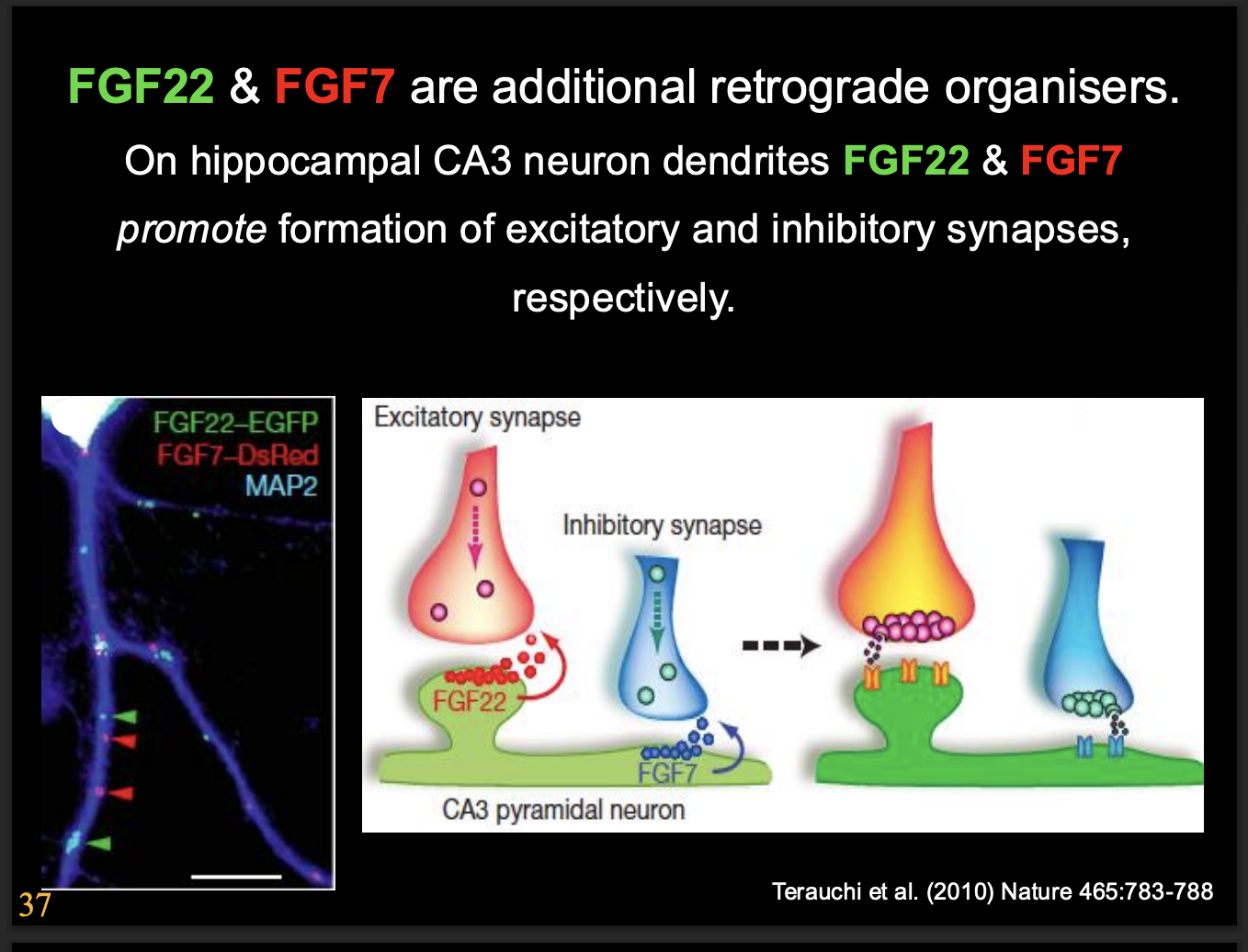
In hippocampal CA3 neurons:
FGF7→ differentiation of inhibitory synapses
shown in knockdown in mice→ increased levels of excitation and susceptibility to epilpetic symaptoms
FGF22→ promotes excitatory synapse
knockdown → decrease susceptibility to epileptic symptoms
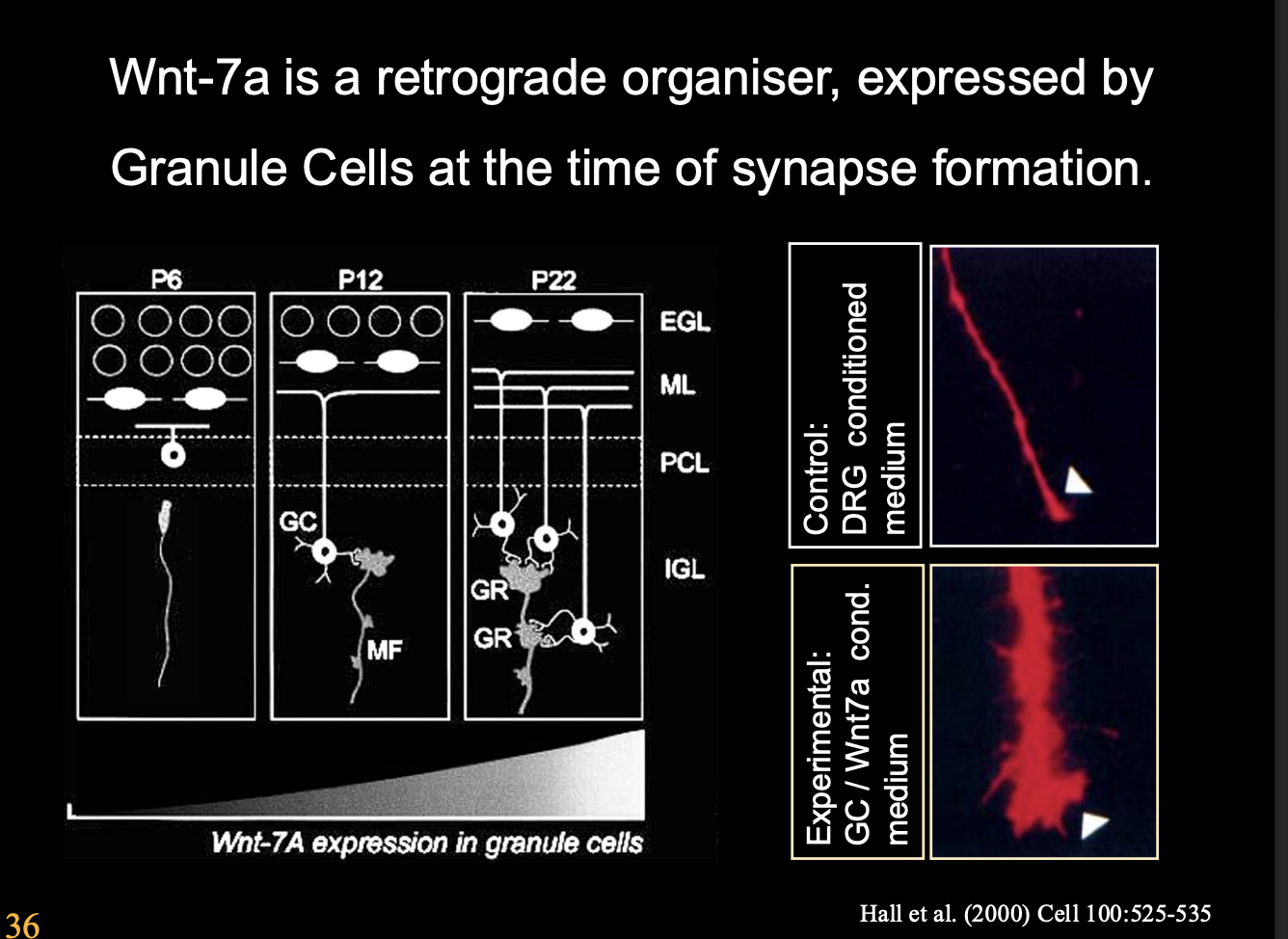
What was the experimental method for this to be found out
take granule cell condition medium
test what induces the fibres to become mossy
when come into contact with postsynpatic parters (granule cells)
How we know the FGF7 and FGF22 specilaisaitons:
can tag them
see where the spots localise to
But the effect in vivo was not as great→ this showed
there are multiple signalling things happening
much more complex interactions
What else is also involved in the formation of excitatory synapses in the CNS
Agrin
Evidence
Agrin knockout mice show a redcution in excitatory central synapses
More research has shown Argin may be part of a
Coincidence detection system
Only when postsynaptic cell’s NMDA receptors are activated
Argin is cleaved by the presynaptically secreted argin-specific protease Neurotrypsin
Bidirectional organization→ what signals are there
Neuoligins and neurexins
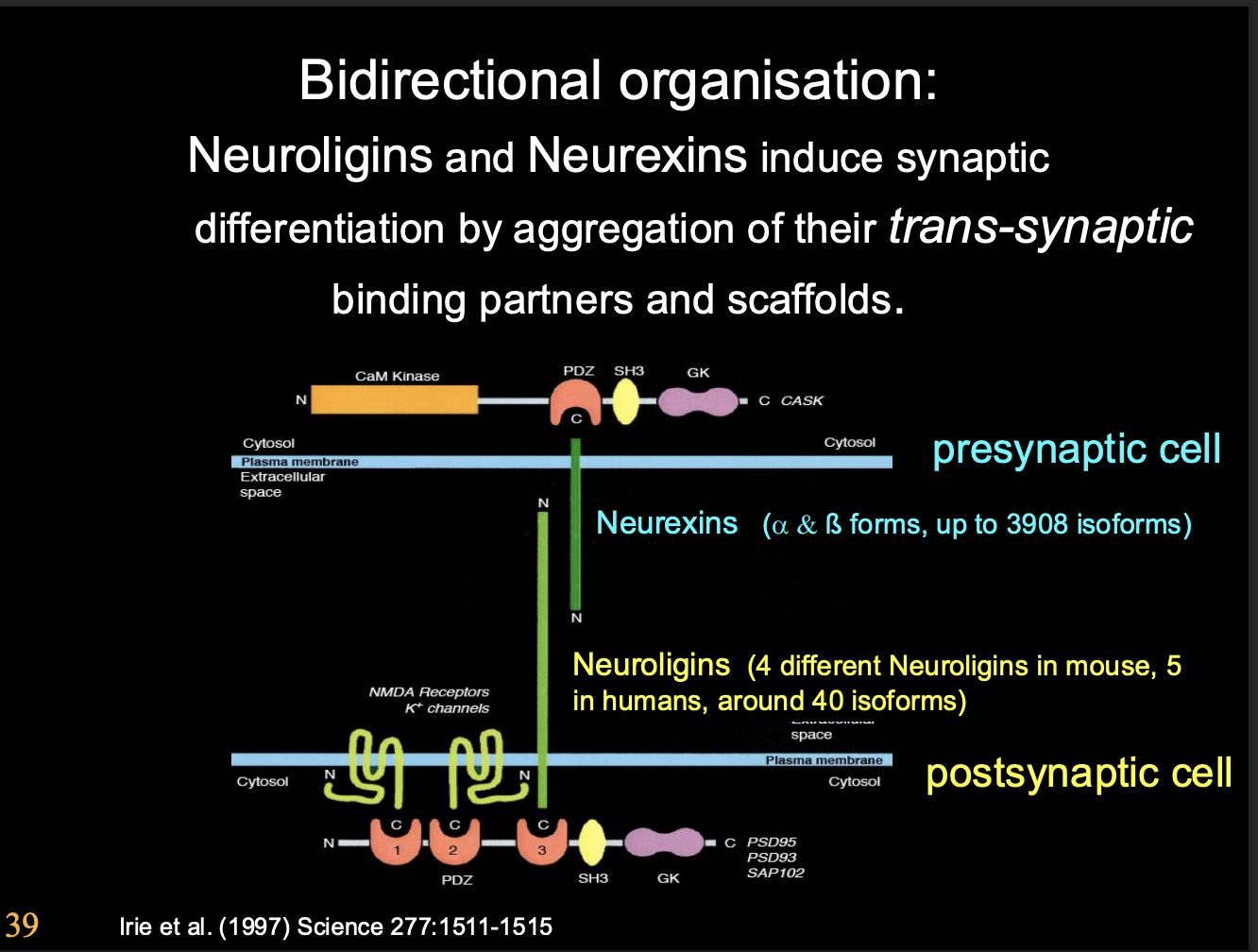
What do Neuoligins and neurexins do
induce synaptic differenetiation
Neurexin→ post synaptic differentiation
Neuroligins→ pre synpatic differentiation
by aggregation of their trans-synaptic binding partners and scaffold (see image)
the genes of neurexins cna be spliced into 1000s of isoforms
forming multiple neuro ligands from just two genes for receptors
HOw do they operate
Transsynaptic binding partners and scaffolds
organise post and prescaffold
anchor cytoskeletal specialisation across the cleft
Evidence: only works if you anchor neurorexin moelcules
so do not act through signals
What do they do
Beads coated with neurexin induce clustering of neurologin
Cause postynaptic differentation
and
Neuroligin can induce presynaptic differentiation through aggregation of neurexins
at the presynatic terminal
overall causes trans-synaptic cell adhesion
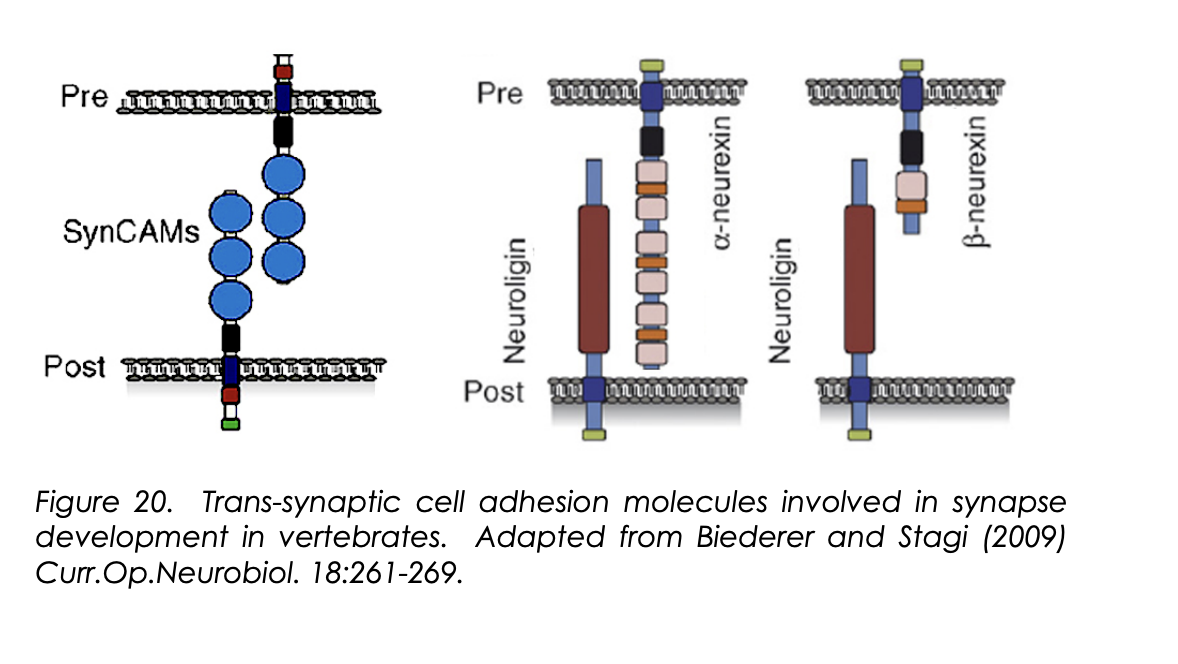
What are trans-synaptic cell adhesion molecules
raft of synamtogenic signals
work as pre and post synaptic terminals to specific common ‘meeting regions’
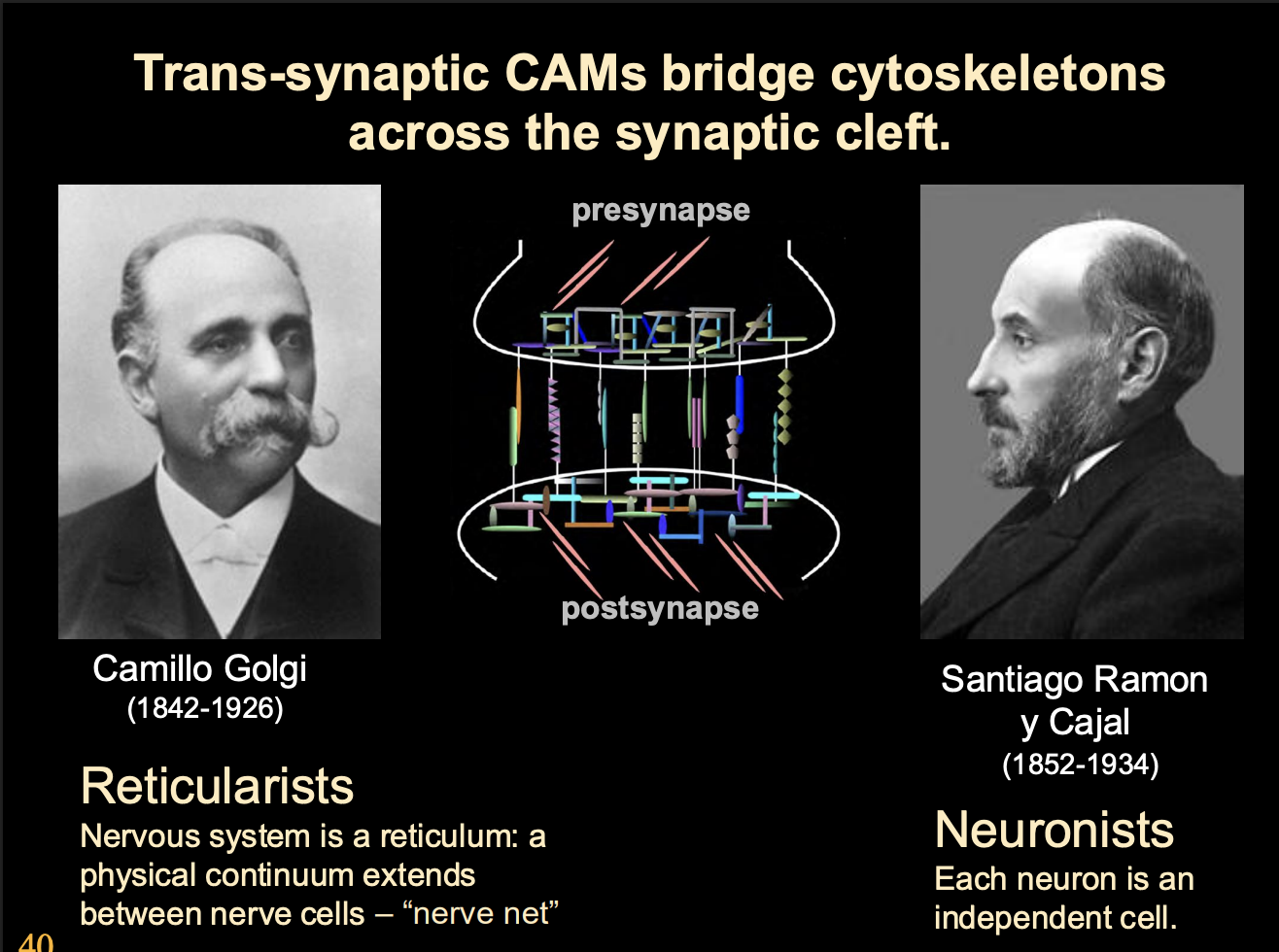
Exmaples of trans-synaptic cell adhesion molecules
EphB-ephrinB
SynCAM
One challenge of these trans-synaptic cell adhesion moelcules
differentiating between what ligand-receptor pairs are capable of under mis-expression conditions
vs
what aspects of nervous system/synapse development they are required for
i.e Neurexin and Neuroligin are shown to be capable of inducing post and pre differentiation respectively
What did mutant mice for genes encoding Neuroligin nad neurorexin show
normal numbers of synapses formed in the CNS
but
had impaired transmission
Suggests:
Neurexin and Neuroligin are likely involved in the maturation of synapses
NOT their induction
A recent study within a population of neurons suggests that the amount of Neuroligin and neurexin may determine…
how many synapses are formed with each of several partner neurons
→ in a compeitive way
Summary
synapse formation requires:
Exchanges of antero and retro-grade signals
between pre and postsynaptic cells
This communication ensures pre an dpost synaptic specialisation are
precise alignment
co-ordinated
it is important to separate autonomous and required processes
Summary of Autonomous vs requirment for pre and post synaptic signalling
Autonomous synaptic differentiation:
Presynaptic terminal:
Transport vesicles carry multiple components – “pre-fabricated” complexes.
Presynaptic release sites can form in the absence of partner neurons.
Postsynaptic terminal:
Pre-patterns exist in muscles (NMJ), less clear for CNS.
Transport vesicles for postsynaptic components ? (controversial – not covered in this lecture)
Requirmnt for pre-postsynaptic signalling
Presynaptic terminal:
Retrograde signals from the postsynaptic cell induce cessation of growth and promote presynaptic differentiation (Wnt7a; FGF22, FGF7, FGF10).
Postsynaptic terminal:
Precise pre- & postsynaptic apposition
Neurotransmitter receptor cluster maintenance Postsynaptic cytoskeleton maintenance