🧪Chemistry⚛️ year 10
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
Physical change
A change in matter that does not form new substances.
Examples of physical changes
Change in
Position
Shape
Size
State
Chemical change/reaction
A change in matter that forms one or more new substances.
Examples of chemical changes
release of light or sound
Formation of a new gas
Change in colour
Disappearance of a solid
Formation of a new solid
Change in temperature
Atom
The smallest particle of an element
Molecule
A group of atoms bonded together
Fixed formula, e.g. O2, H2O
Chemical bond
An attractive force that holds two atoms together
Mixture
A combination of substances that can be physically seperated.
Element
Any of the basic substances on the periodic table, such as oxygen (O2), iron (Fe) and gold (Au).
Compound
Made up of two or more different types of atoms bonded together, such as carbon dioxide (CO2) and sodium chloride (NaCl).
Chemical equation
Reactants—>Products
e.g. sodium + oxygen → sodium oxide
Reactants
The substances that react with each other
Products
The new substances formed by a reaction
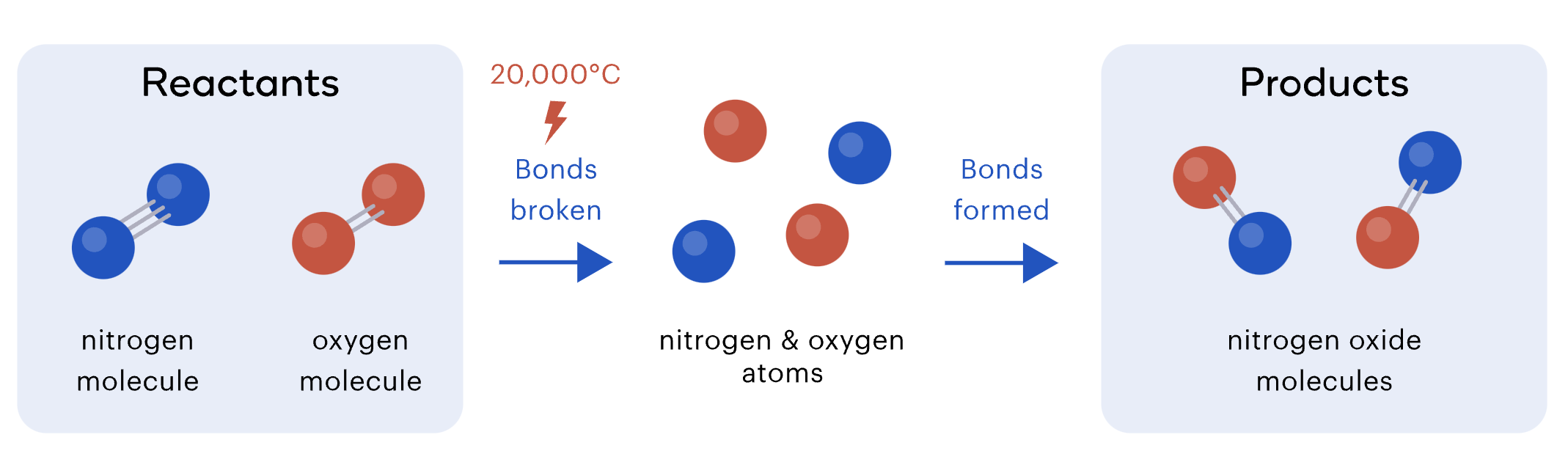
The re-arrangement of atoms
During a chemical reaction, some of the chemical bonds between atoms are broken and new bonds are formed.
This re-arrangement of atoms is what produces a new substance.
The same elements are present after a reaction – they're just arranged in a new way.
Metal atom
1, 2, or 3 electrons in outer shell
Non-metal atom
5, 6 or 7 electrons in outer shell
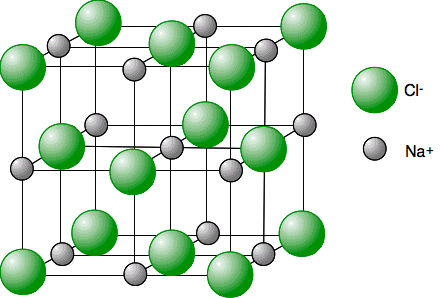
Lattice
Continuous arrangements of bonded atoms in regular patterns.
Ratio of elements, e.g. NaCl, Au
Why do elements bond together?
Atoms form chemical bonds to obtain full valence shells.
By bonding together in chemical reactions, atoms can reach a more stable state.
Ions
Charged particles formed when atoms either lose or gain electrons
Cations
Positively charged ions formed by the loss of electrons
Anions
Negatively charged ions formed by the gain of electrons
Ionic bonds
The transfer of electrons from one atom to another results in two ions with opposite charges. The attraction between these opposite charges is what makes an ionic bond.
Occurs between metals and non metals
Metal cations
Non-metal anions
Predicting ionic compounds
E.g: Aluminium + Chloride
Aluminium- Metal, charge of 3+
Chloride- Non-metal, charge of 1-
This means you need three negative charges to balance out the positive charge of three.
(Al³⁺)+ (Cl⁻)+ (Cl⁻)+ (Cl⁻)
= AlCl₃
metals are always written before non-metals
Metallic bonding
When metals bond with metals, they donate their valence electrons into a common pool.
This results in metal cations floating on a sea of delocalised electrons
The positively charged metal ions (cations) are held together by the attraction to this sea of delocalized electrons.
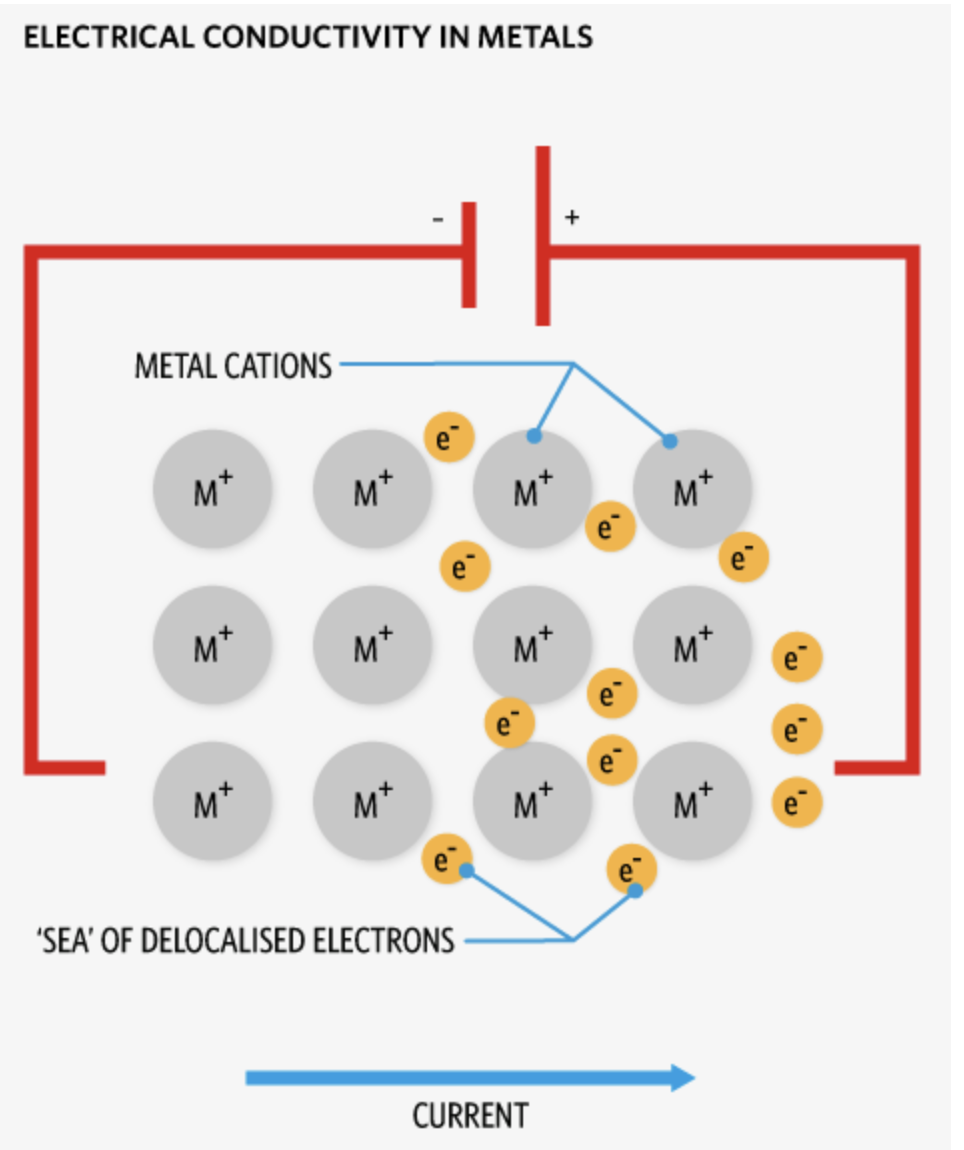
Properties of metals explained by metallic bonding- Electrical Conductivity
Delocalised electrons can move freely through the metal- a flow of electrons is an electric current
Properties of metals explained by metallic bonding- Heat Conductivity
The delocalised electrons carry thermal energy (heat) through the metal quickly and easily.
Properties of metals explained by metallic bonding- Shine
Delocalised electrons move quickly so that light can reflect off all surfaces of the metal.
Covalent bonding
When non-metals bond with non-metals, they share a pair of electrons between atoms.
Each atom contributes one or more electrons to form a shared pair.
One pair represents two total electrons
This sharing allows both atoms to achieve a stable electron configuration (a full outer shell).
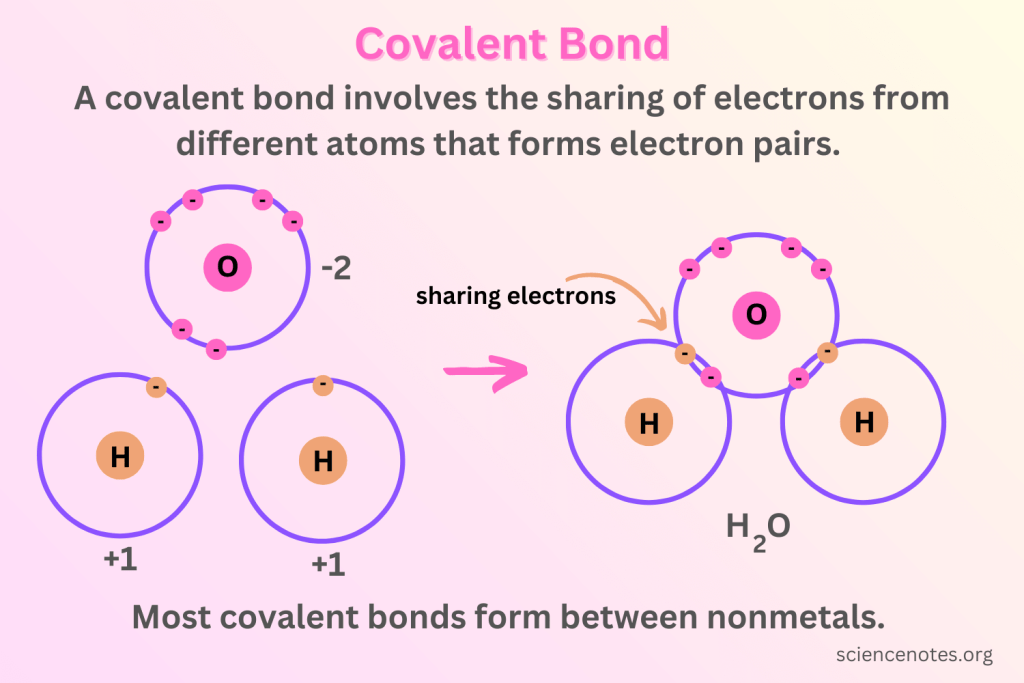
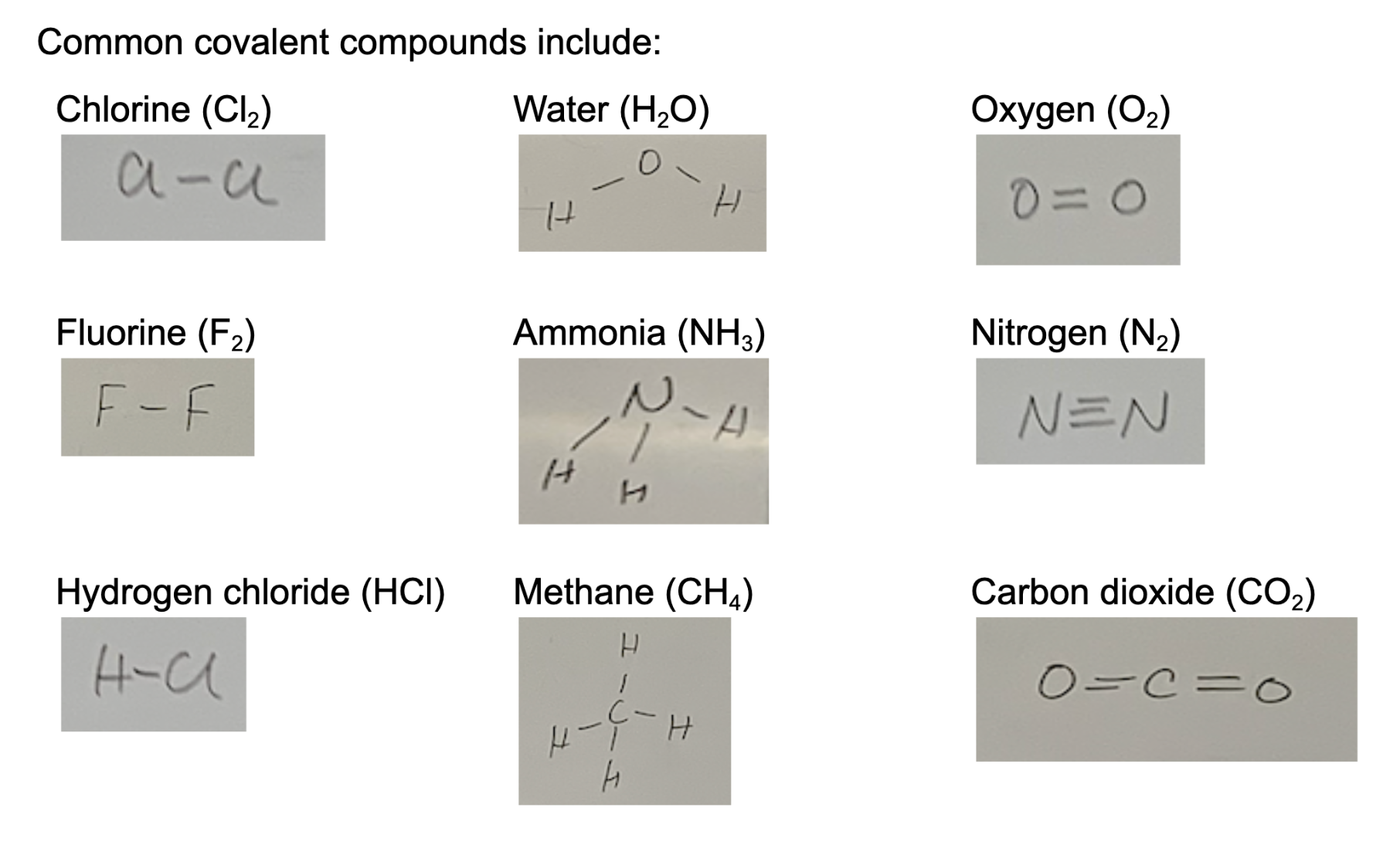
How to represent covalent bonding
F-F, H-H, H-O-H
The number of lines between the element symbols represent the number of bonds
Bonds of common elements
H, F, Cl- single bond ( two electrons are shared)
O- double bond(four electrons are shared)
N- triple bond( six electrons are shared)
C- quadruple bond( 8 electrons are shared)
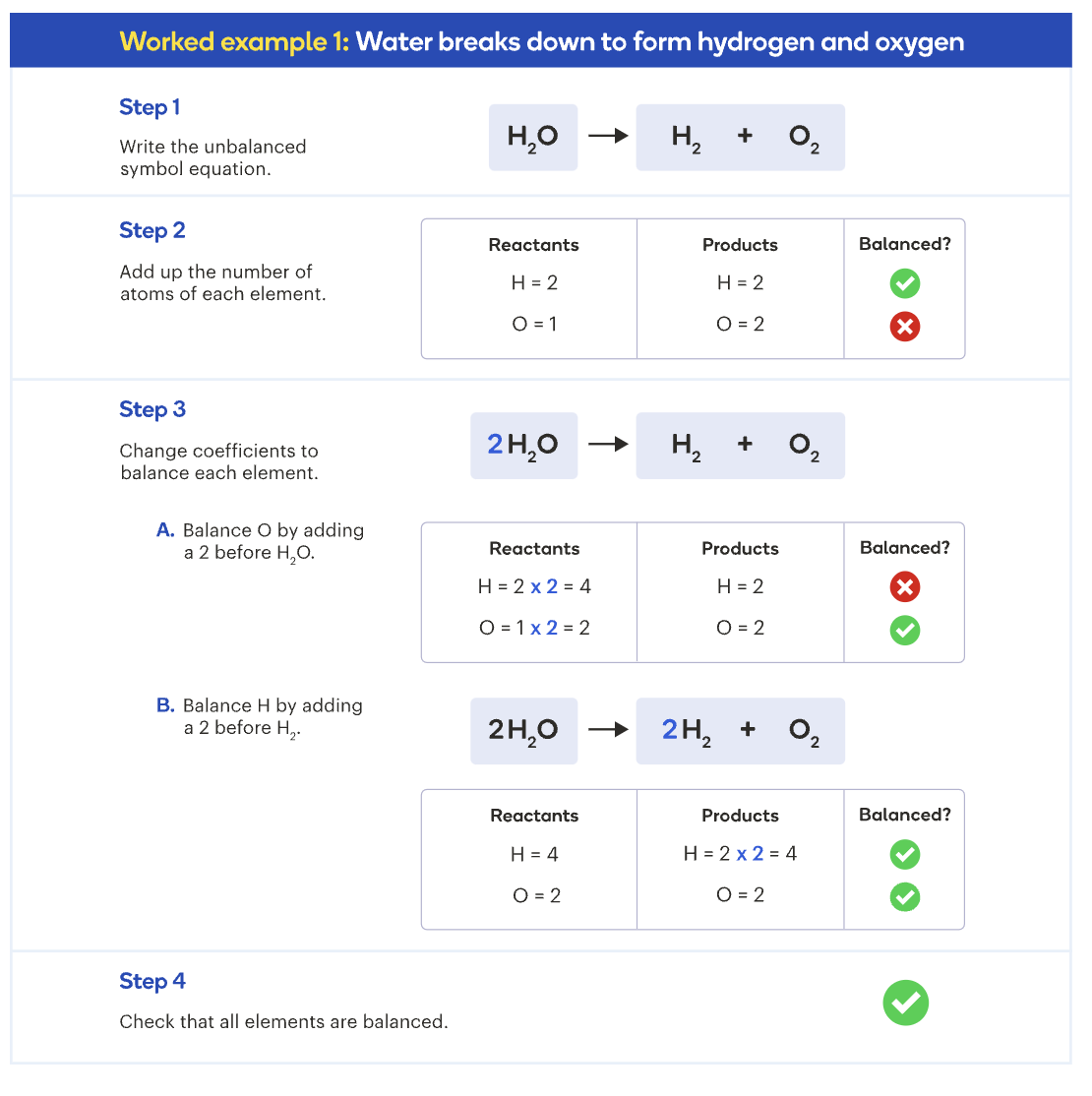
Law of Conservation of Matter
In a chemical reaction, atoms are not created or destroyed.
Total number of atoms of each element remains the same before and after the reaction.
The bonds of the atoms are just rearranged in different ways, forming new substances.
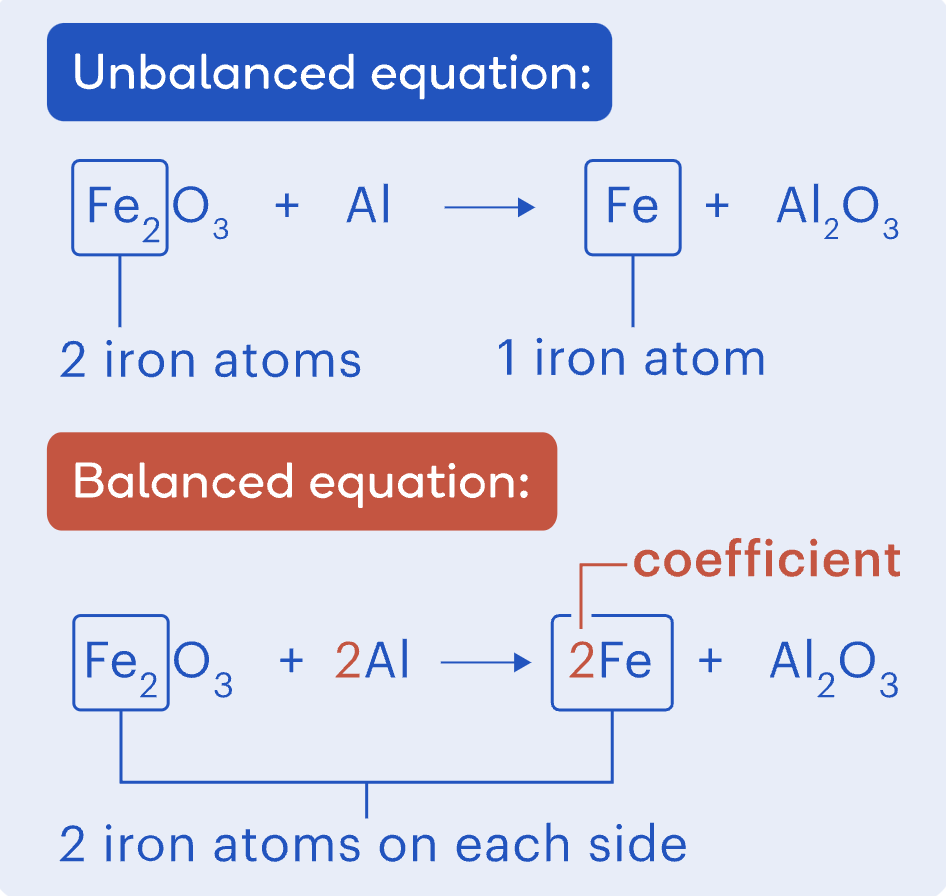
Unbalanced Equations
An equation is unbalanced if the number of atoms of each element is not the same on both sides.
Example (Unbalanced Thermite Reaction):
Fe₂O₃ + Al → Fe + Al₂O₃
The number of Fe and Al atoms do not match.
Balancing equations
An equation is balanced when the number of each type of atom is the same on both sides.
To balance an equation:
Adjust the numbers in front of chemical formulas (coefficients).
Example (Balanced Thermite Reaction):
Fe₂O₃ + 2Al → 2Fe + Al₂O₃
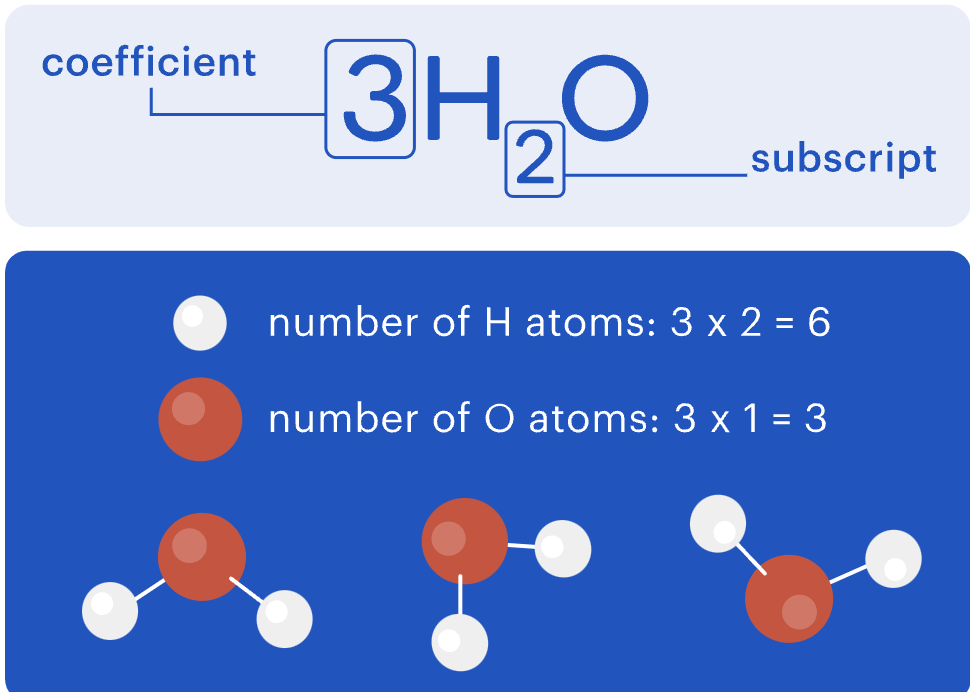
Subscripts and coefficients in chemical equations
When balancing an equation, you can’t change the subscript numbers, as this shows how the molecule is naturally formed.
You can only adjust the coefficients, which change the number of elements or compounds in a chemical equation.
To find the amount of each type of atom, multiply the coefficient by the subscript
How to balance equations
Composition reactions
Two or more reactants combine to form a single product.
General form: A + B → AB
Examples: N2+3H2→2NH3, 2Mg+O2→2MgO
multiple reactants, single product
Decomposition reactions
One reactant breaking down into multiple products
General form: AB→ A + B
Examples: 2H2O→2H2+O2, CaCo3→ CaO+CO2
Single reactant, multiple products
Displacement reaction
One element replaces another in a compound
General form: AB+C→BC+A
Examples Zn+CuSO4→ZnSO4+Cu, Cu + AgSO4→2Ag+CuSO4
One metal dissolving, another coming out of solution
Often two metals involved
Neutralisation reaction
Acids and bases reacting together
General form: Acid + base→water + salt
Examples: HCl+ NaOH→H2O+NaCl, H2SO4+Mg(OH)2→2H2O + MgSO4
Acid, base
Precipitation reactions
Two solutions are mixed and a solid forms
General form: AB+CD→ AD+CB (one product is solid)
Examples: Pb(NO3)+2KI→ PbI2+2KNO3
Solid forms from mixing solutions
Three main types of chemical bonding
metal + metal → metallic bonding
metal + non-metal → ionic bonding
non-metal + non-metal → covalent bonding
Collision theory
The idea that the re-arrangement of atoms requires collisions between the reactant particles.
It's only when the particles are in contact that new bonds can form to make the products.
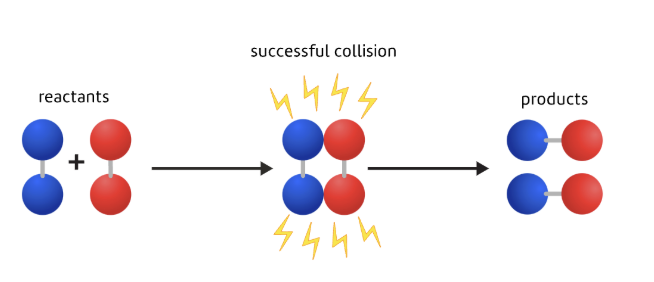
What a successful collision involves
Particles colliding with both
the right orientation, and
enough energy, or speed, to break their bonds
If both conditions aren't met then the particles will just bounce off each other without reacting.
Rate of a reaction
How quickly reactants are converted into products.
The higher the frequency of successful collisions, the higher the reaction rate.
What you need to increase the rate of a chemical reaction:
surface area
temperature
concentration
Surface area
Surface area is the total outside area of a three dimensional solid.
As a solid is broken into smaller prices, the surface area increases.
When the surface area is increased, the chance of reactant particles colliding increases, so there are more reactions and the reaction is faster.
Temperature
When the temperature is increased, the average kinetic (movement) energy of the particles is increased, so particles move faster.
More collisions occur, and more particles have enough energy to react. This means there are more reactions and the reaction is faster.
Concentration
Concentration is how many particles there are per unit volume.
When the concentration is increased, the chance of reactant particles colliding increases, so there are more reactions and the reaction is faster.