Coordination Compounds
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Dative Bond (Coordinate Bond)
One of the atoms supplies both the shared electrons to the covalent bond.
Ligands
A molecule that donates a lone pair to a metal
coordination compound
consists of a transition metal with attached ligands and counterions
Which are the counter ions? [Fe(CO)4Cl2]Br
The ions outside the square brackets
Br- in this example
How do you determine charge on the TM in a complex?
Charge on Ligand + Charge on Metal = Charge on complex
Charge on ligands (lewis structure or tables)
Charge on complex based on the counterions
Solve for Charge on metal
oxidation state
The charge of the TM ion (e.g., +1, -2, etc.)
electron configuration of TM ions
the 3d fills before 4s (or 4d before 5s)
d electrons on a metal in a complex
the total number of d electrons given the oxidation state of the metal
Total electrons on TM in a complex
d electrons + 2 electrons per ligand binding site
Denticity
The denticity of a ligand refers to the number of donor atoms it has, and therefore the number of bonds it can make to the transition metal.
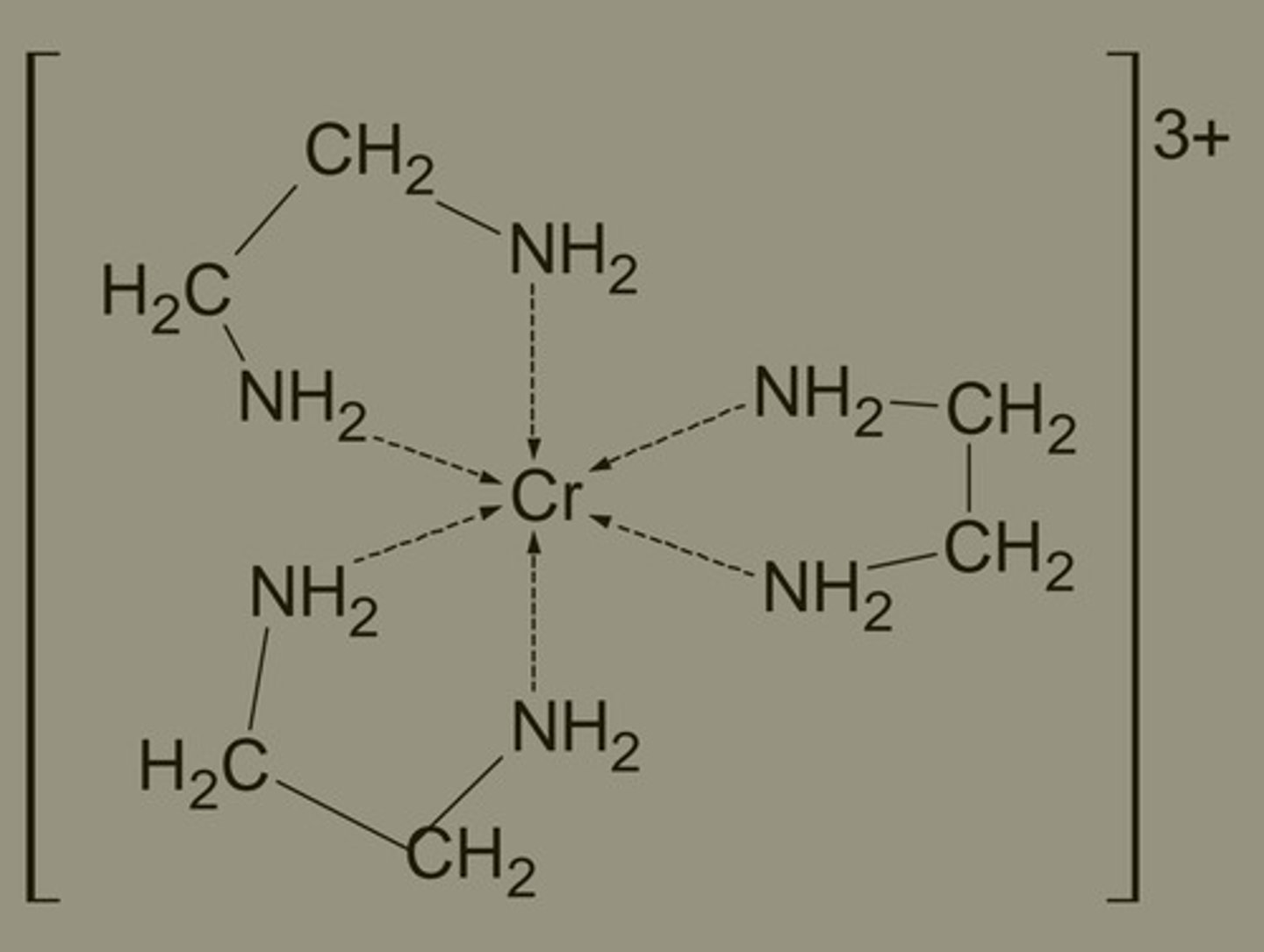
denticity of ethylene diamine (en)
NH2CH2CH2NH2
bidentate
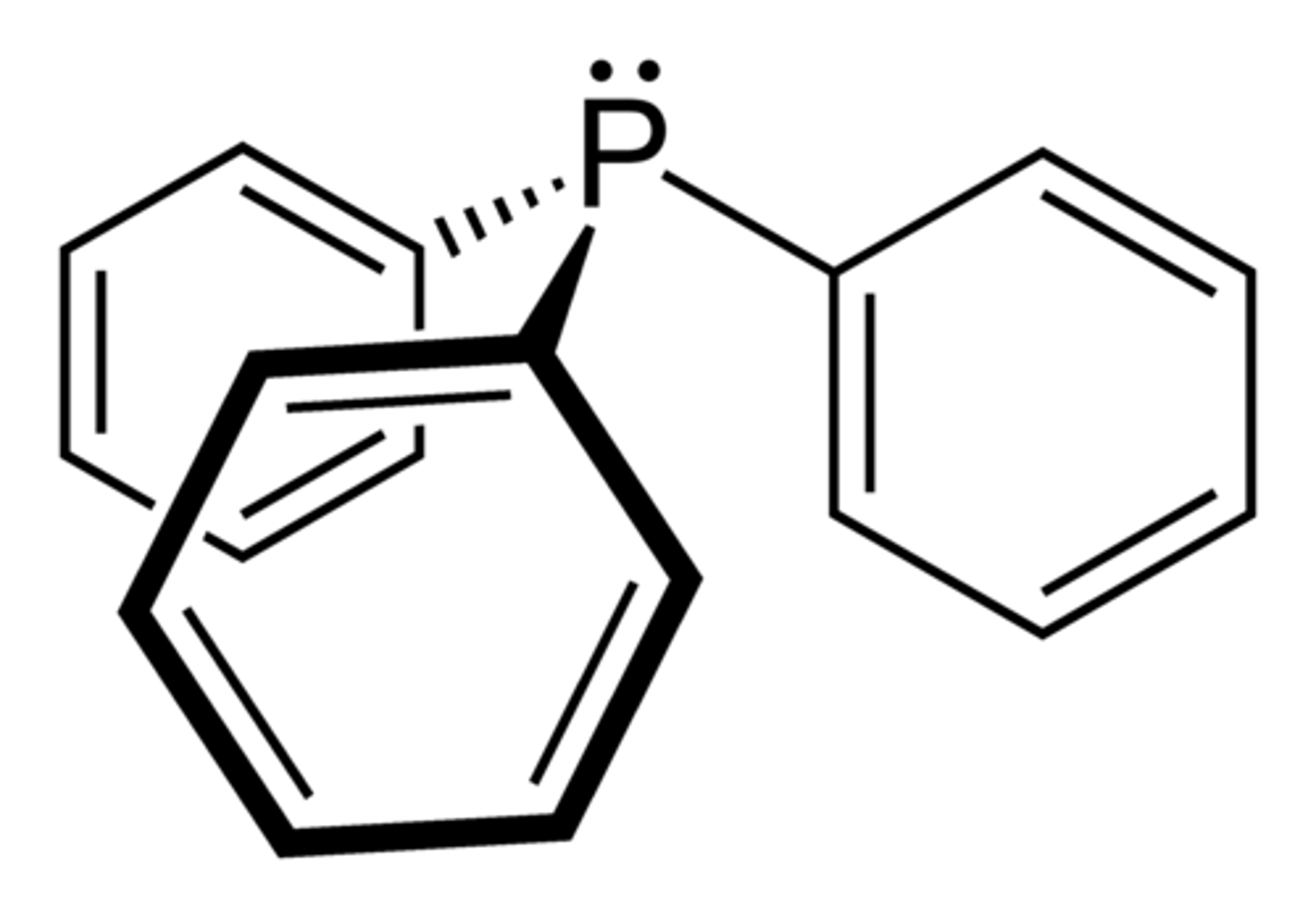
denticity of Ph3P
monodentate
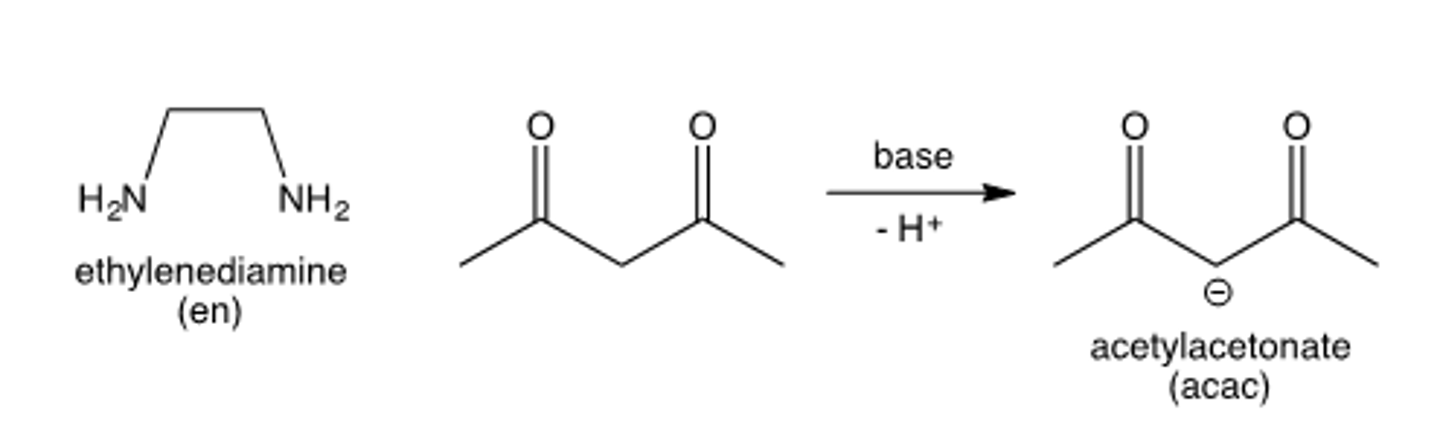
denticity of acetoacetate (acac)
bidentate
hapticity
# of atoms of a ligand attached to a metal atom
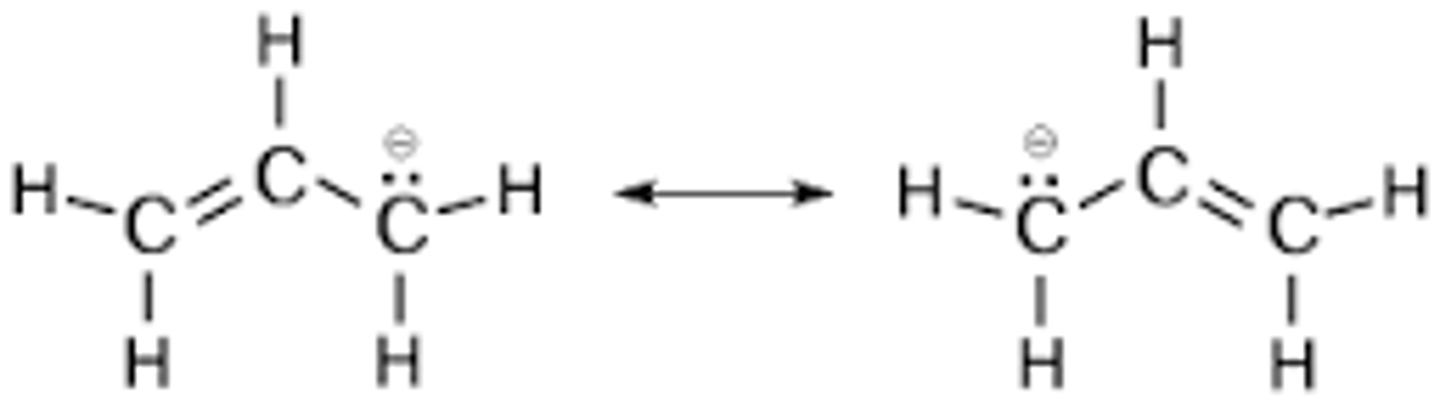
hapticity of allyl ligand (CH2CHCH2)-
eta-3
hapticity of benzene
eta-6
Ligand names: NH3
ammine
Ligand names: water
aqua
Ligand names: Br-
bromo
Counterion names: Br-
bromide
Ligand names: CN-
cyano
Ligand names: CO
carbonyl
Ligand names: PPh3
triphenylphosphine