quantifying equilibria
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
equilibrium constant K
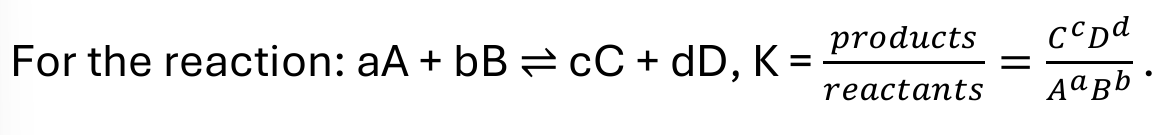
when are reactants and products favoured
reactants when K < 1, products when K > 1
Kp
equilibrium constant expressed as partial pressures. only used for reactions taking place completely in the gas phase
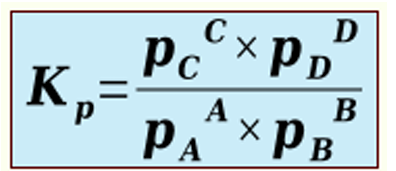
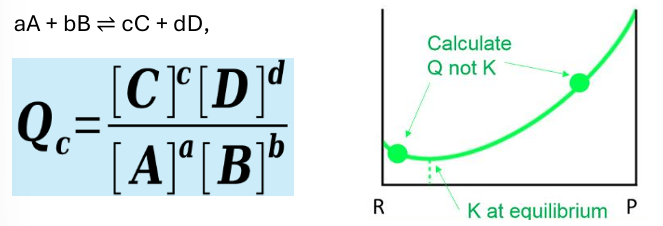
reaction quotient Q
relation to K
the relative amounts of products and reactants at any point during a reaction
Q = K at equilibrium
Q > K, reaction proceeds to left
Q < K reaction proceeds to right
non-equilibrium gibbs energy
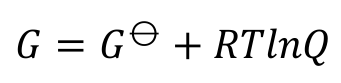
gibbs energy at equilibrium
Q = K and ΔG = 0 so ΔG⦵ = -RTlnK
as ΔG = ΔH - TΔS, ΔH - TΔS = -RTlnK
what is solubility
the amount of substance (mol) that can be dissolved in a given amount of substance (dm3)
chemical equation for dissolving
MX (s) ⇌ M+ (aq) + X- (aq)
solubility product
Ksp = [M+ (aq)][X- (aq)]
large Ksp = high solubility
concentration and activity of solids
both 1