Molecular Geometry (and Electron Geometry)
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
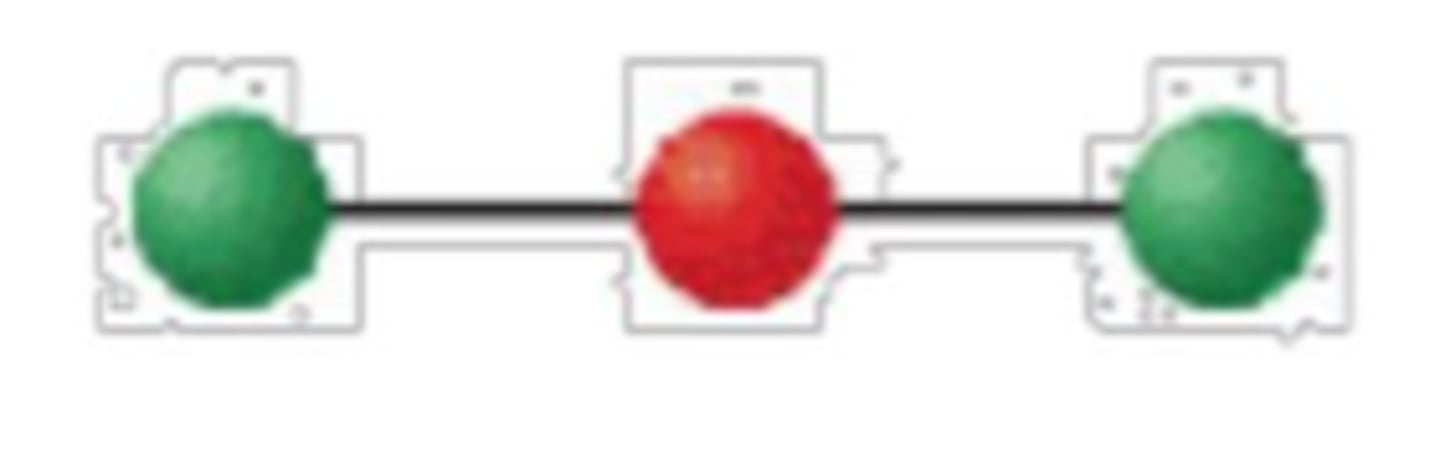
2 electron groups, 0 lone pairs
linear
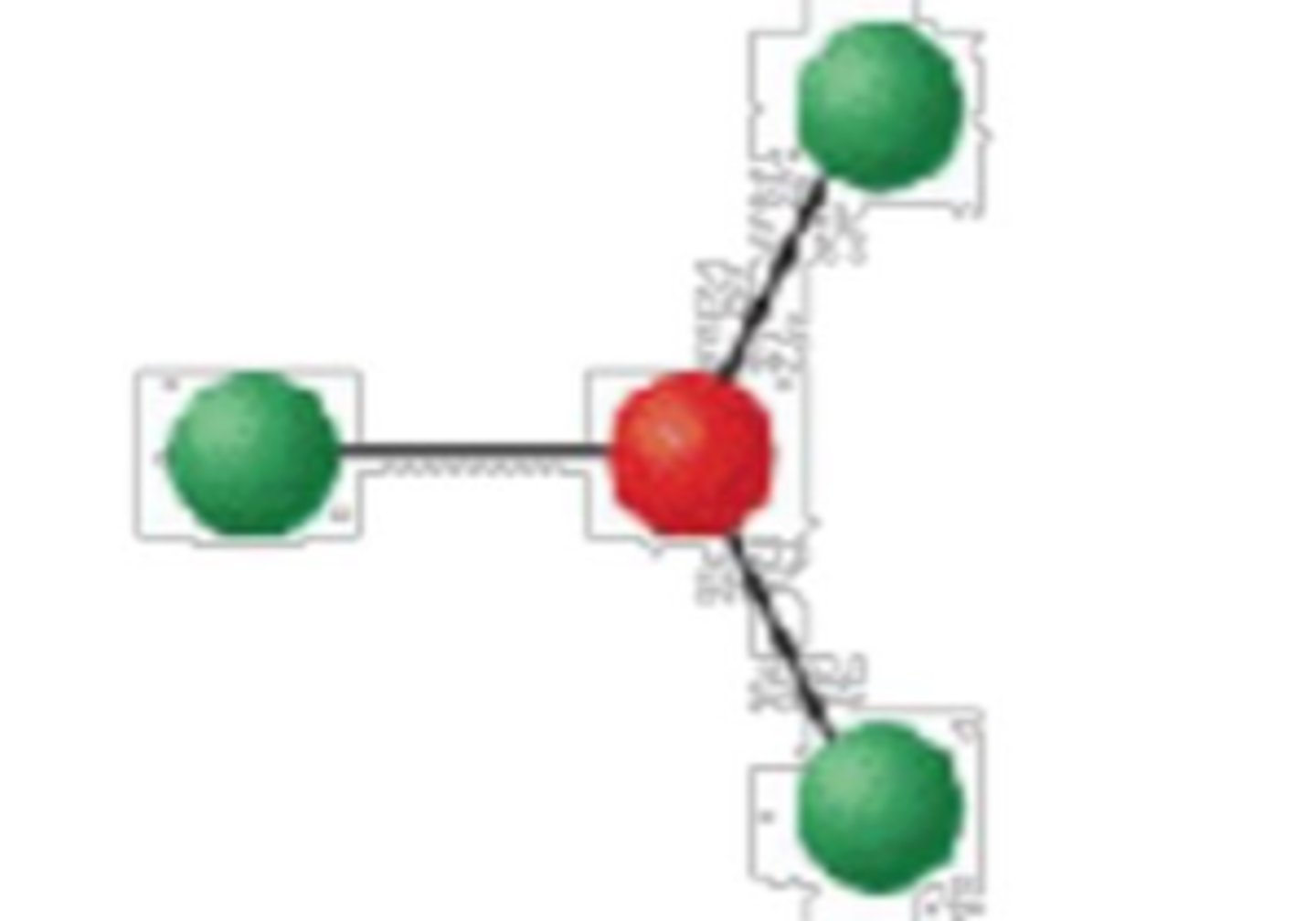
3 electron groups, 0 lone pairs
trigonal planar; 120°
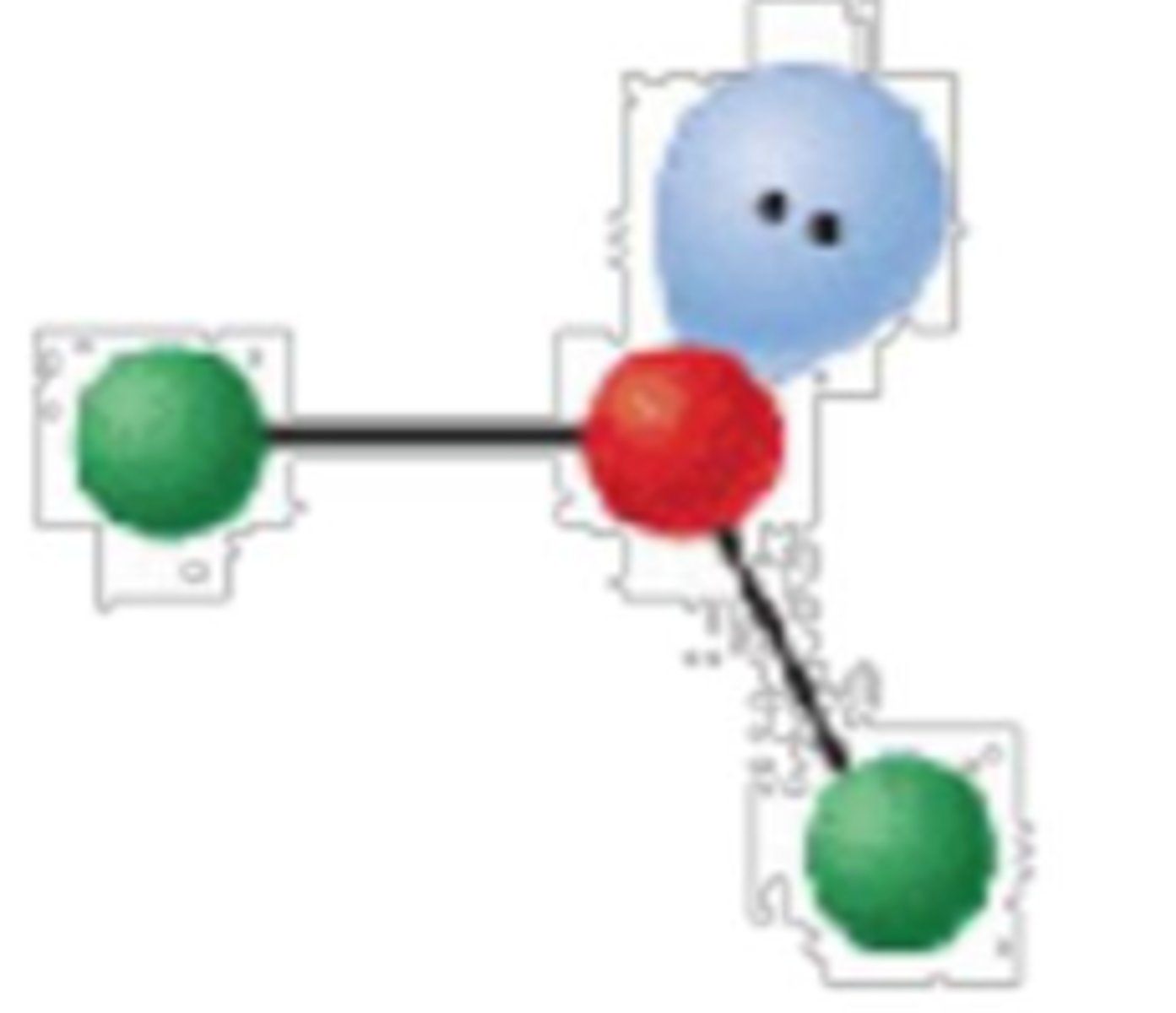
3 electron groups, 1 lone pair
bent or angular; <120°
4 electron groups, 0 lone pairs
tetrahedral; 109°
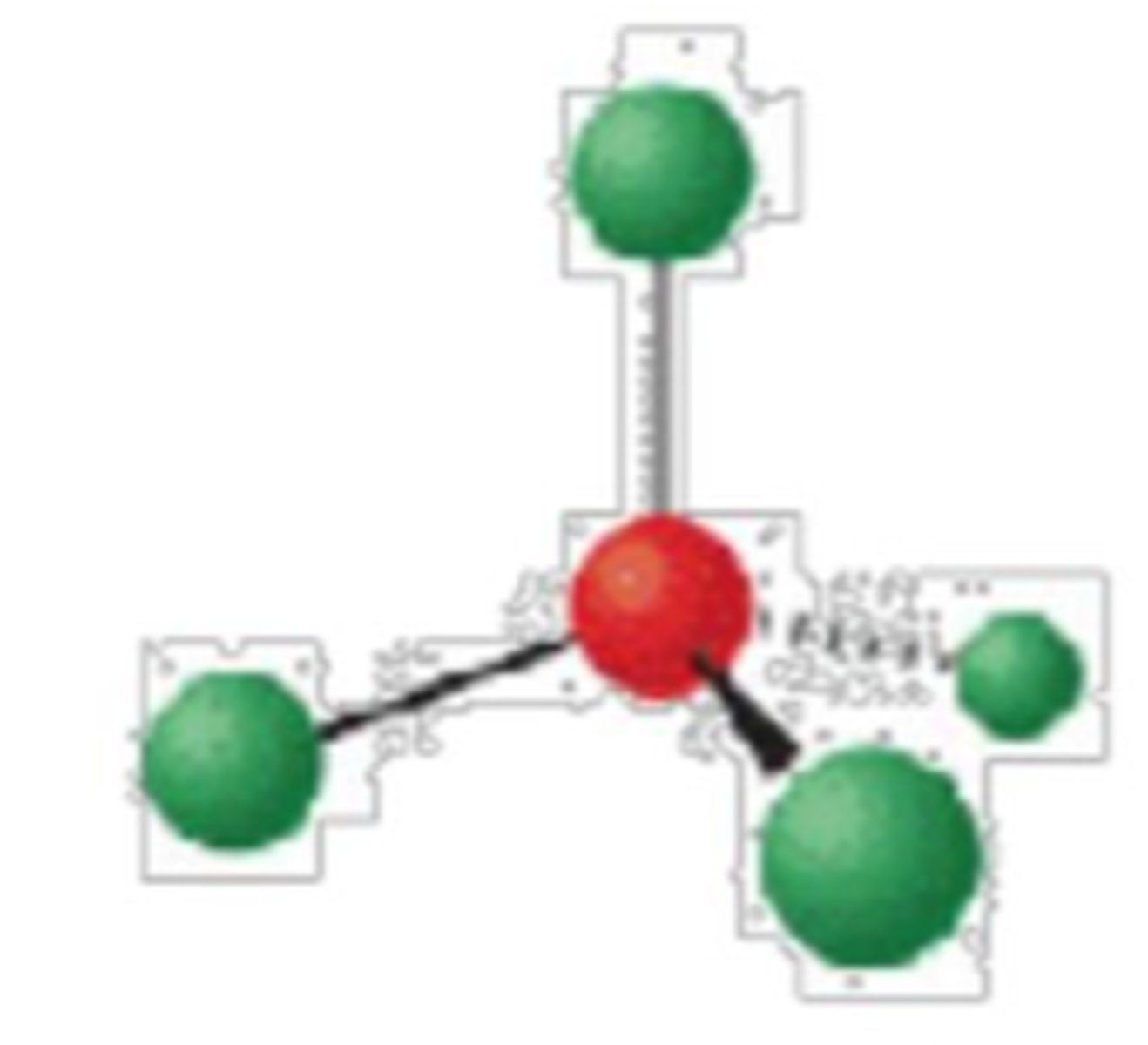
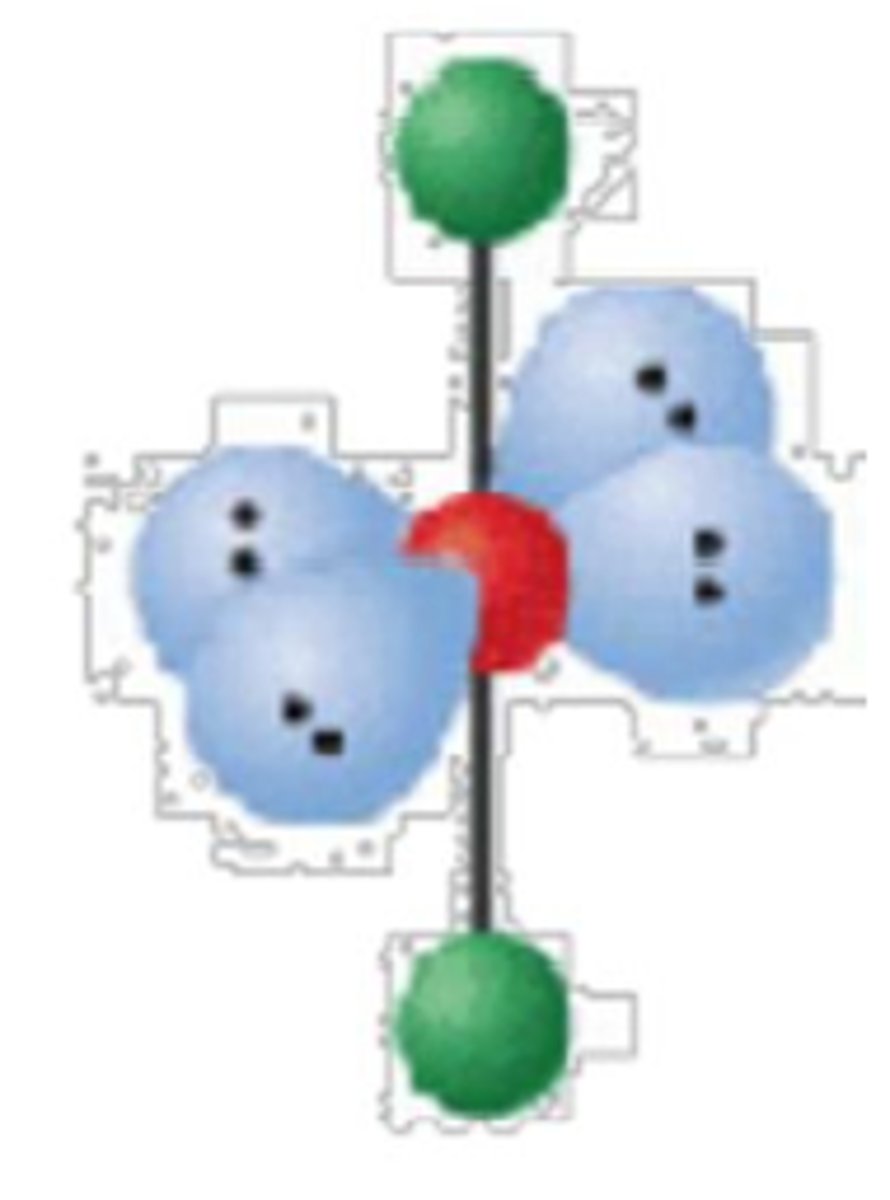
4 electron groups, 1 lone pair
trigonal pyramidal; 109°
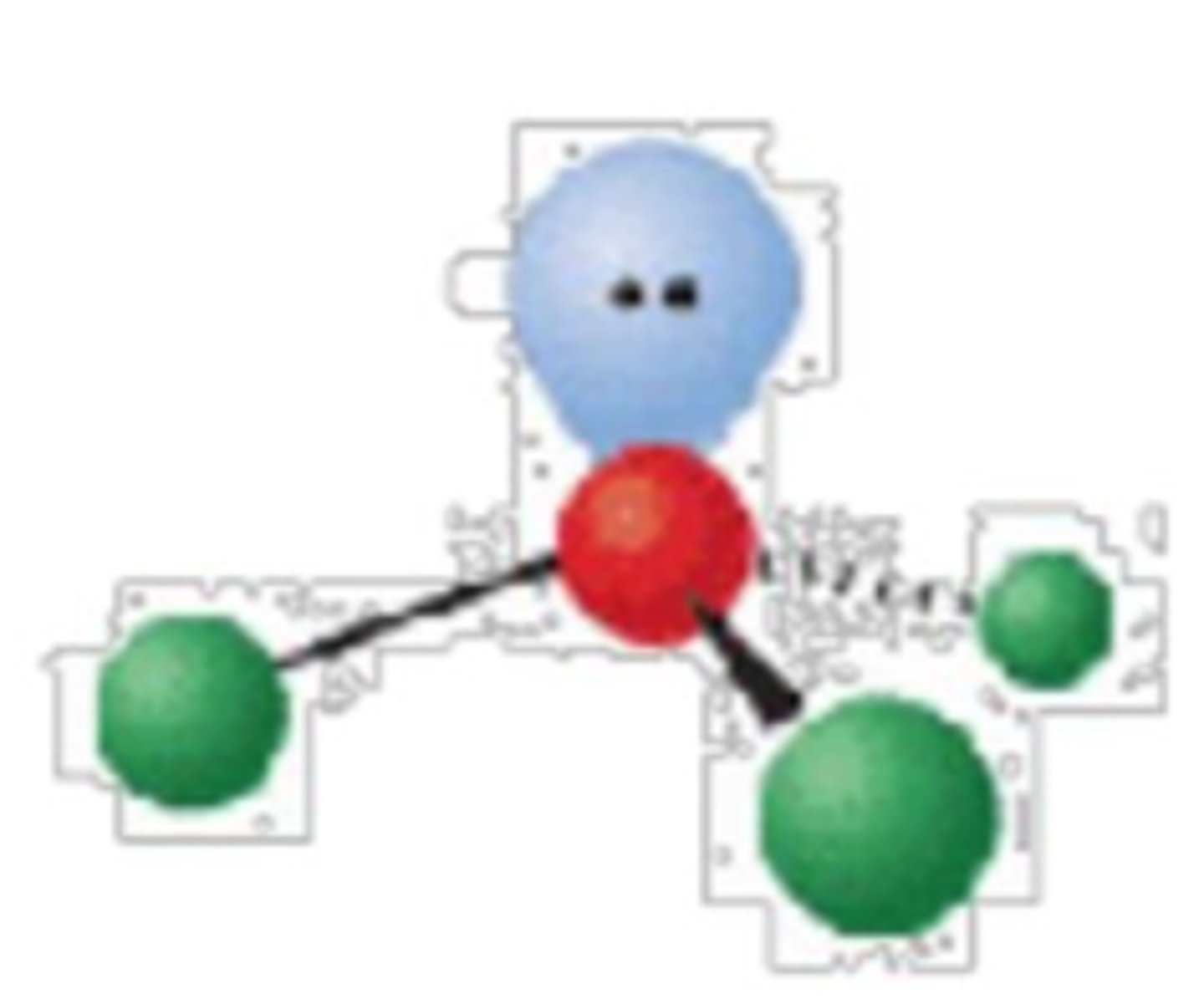
4 electron groups, 2 lone pairs
bent; <<109°
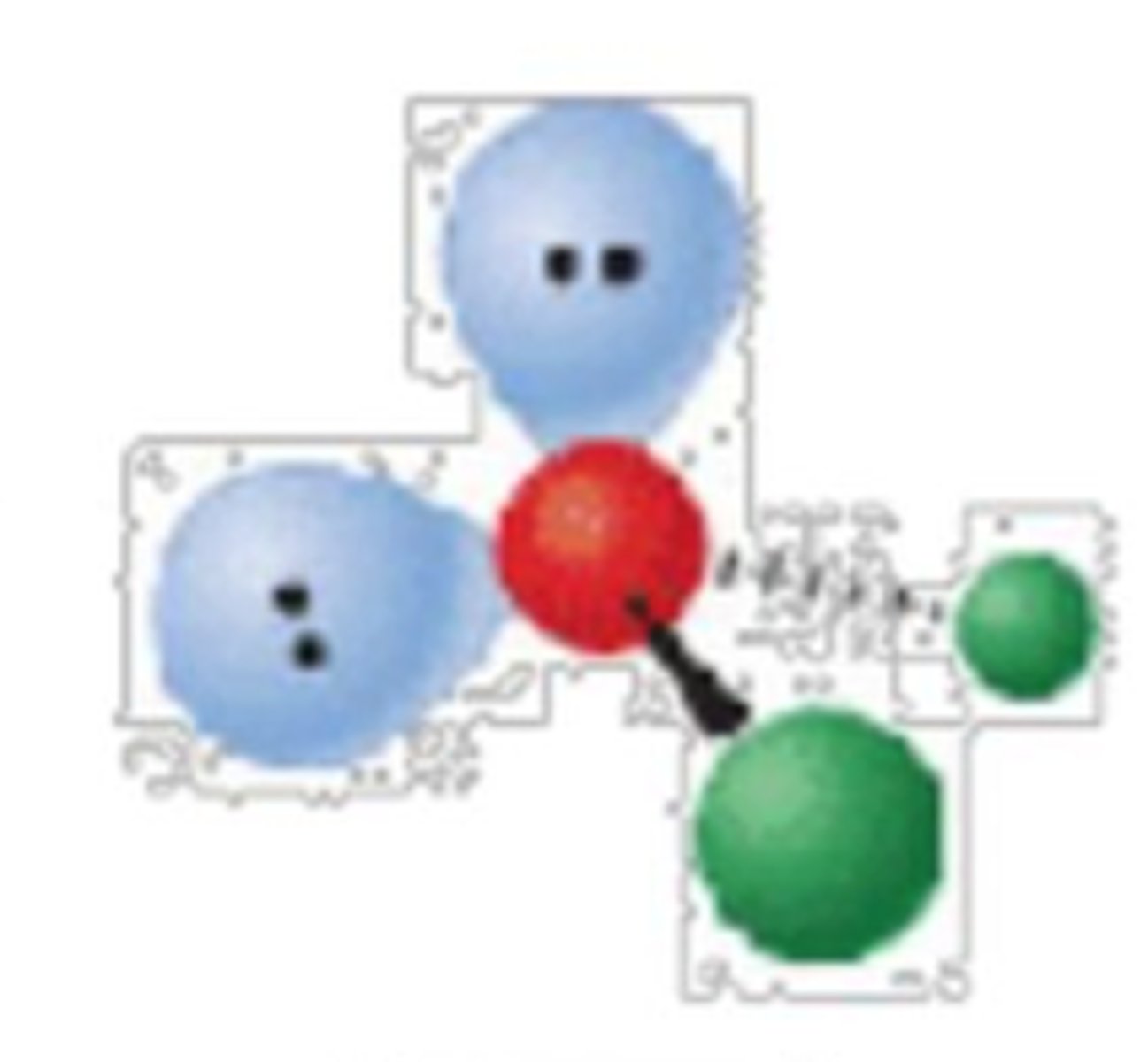
5 electron groups, 0 lone pairs
trigonal bipyramidal; 120° and 90°
5 electron groups, 1 lone pair
seesaw; <120° and <90°
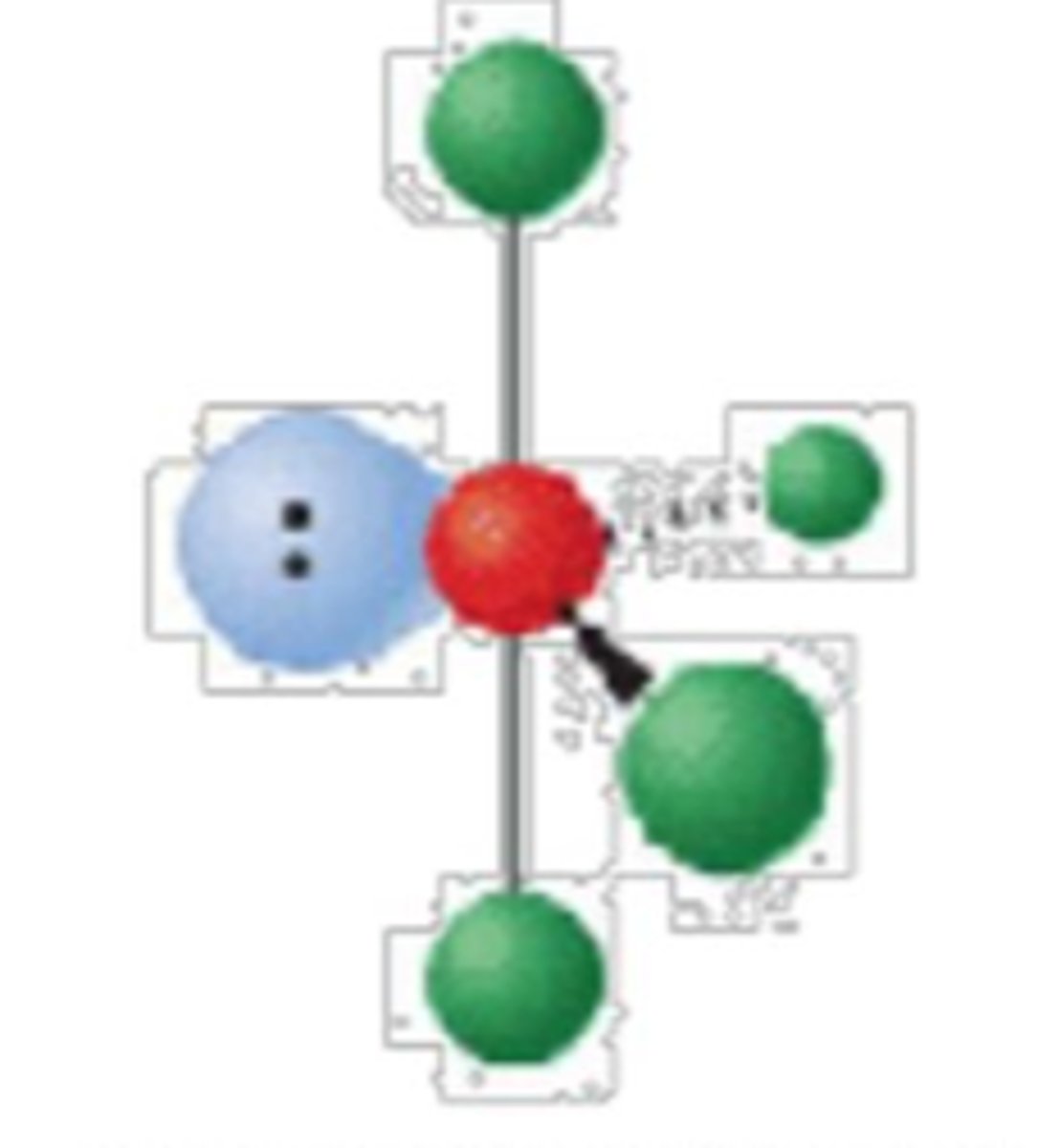
5 electron groups, 2 lone pairs
T-shape; <90°
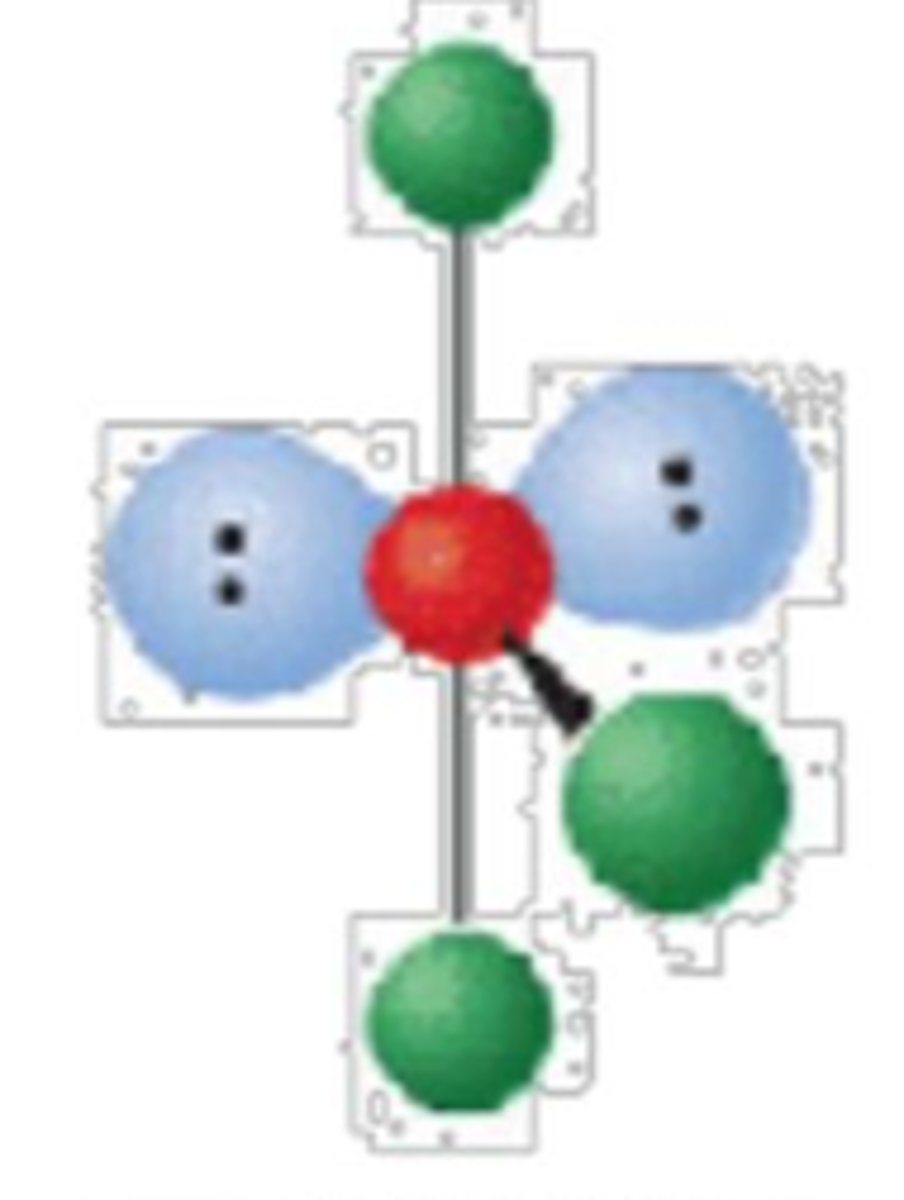
5 electron groups, 3 lone pairs
linear; 180°

6 electron groups, 0 lone pairs
octahedral; 90°
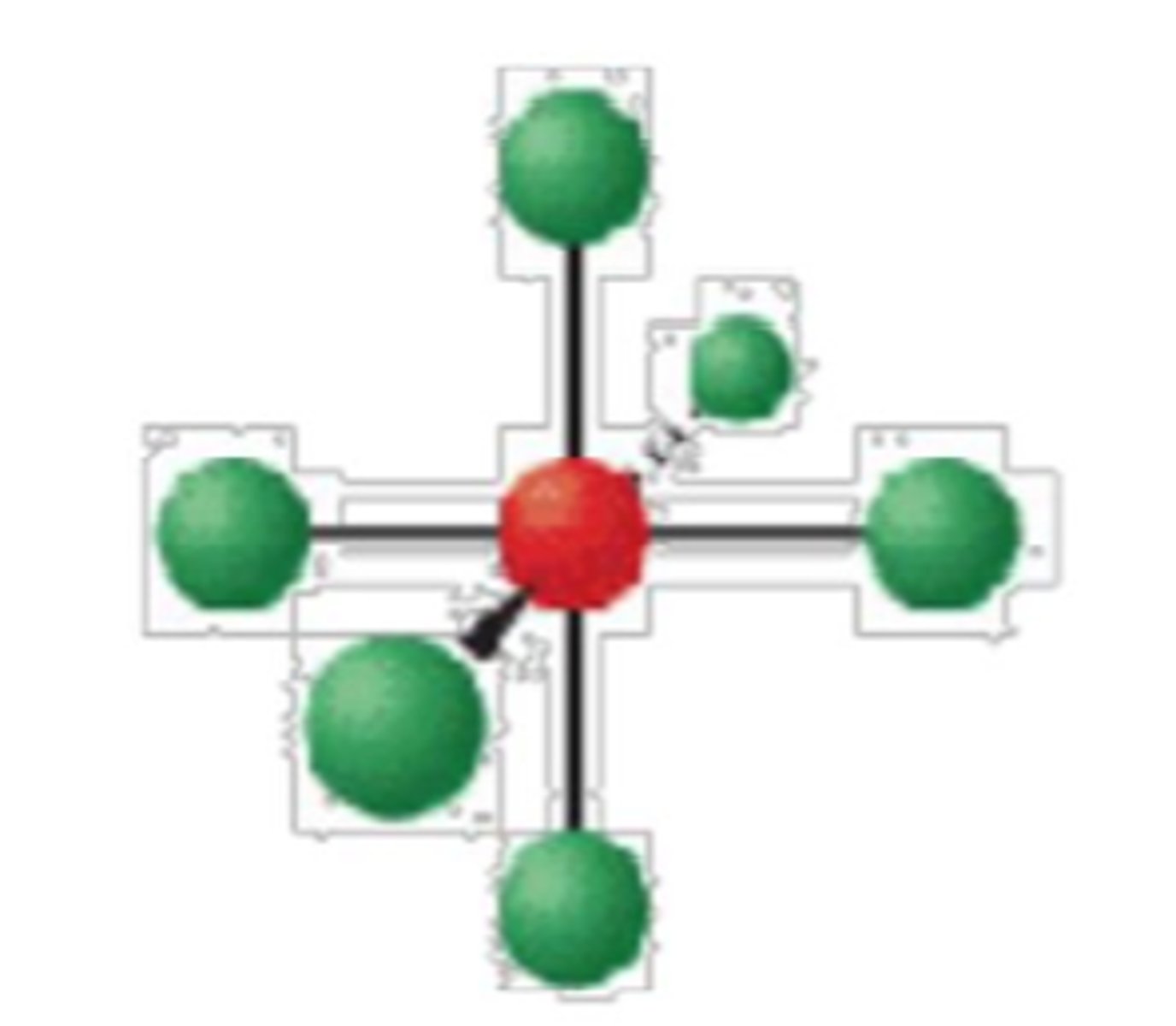
6 electron groups, 1 lone pairs
square pyramid; <90°
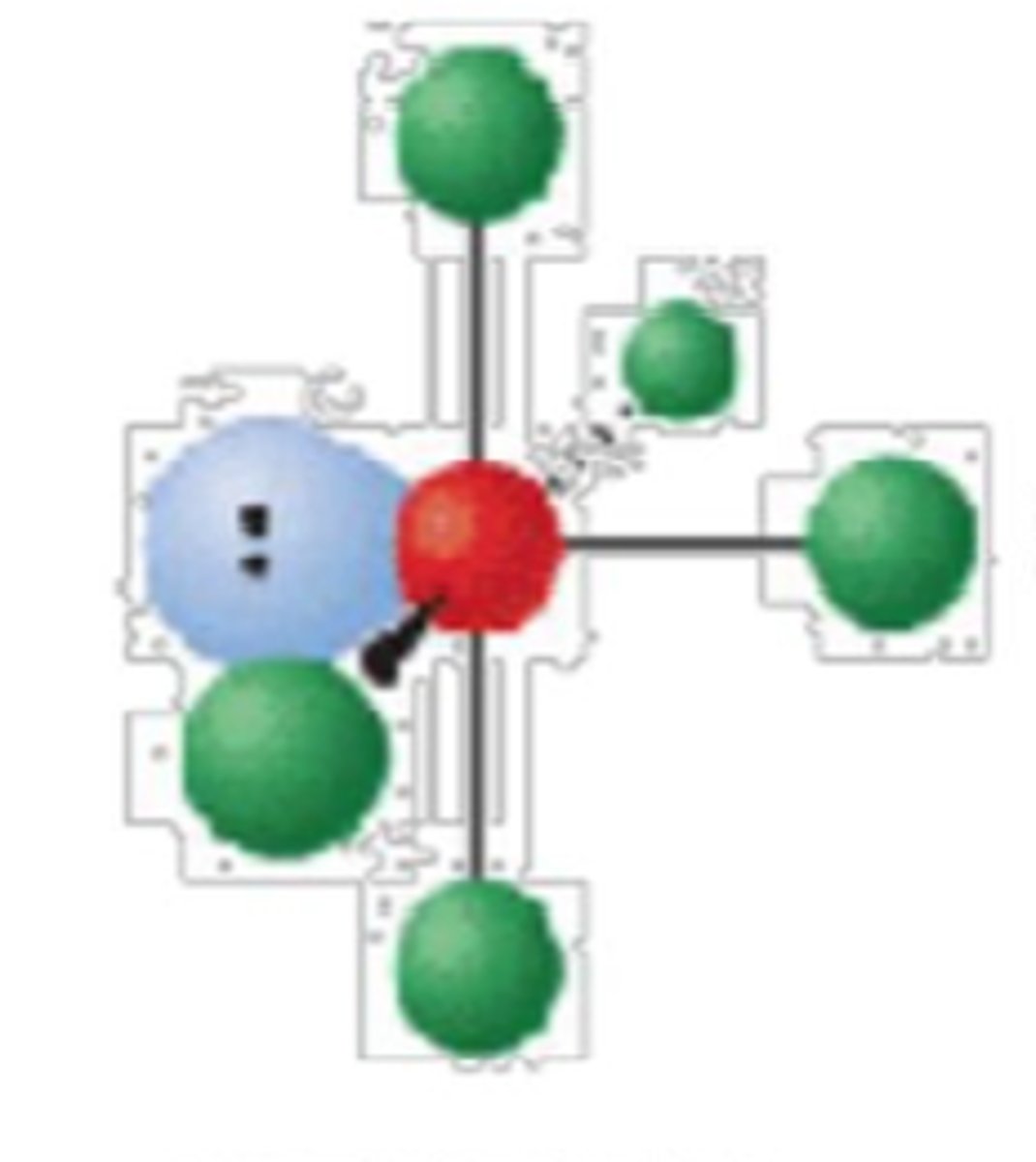
6 electron groups, 2 lone pairs
square planar; 90°
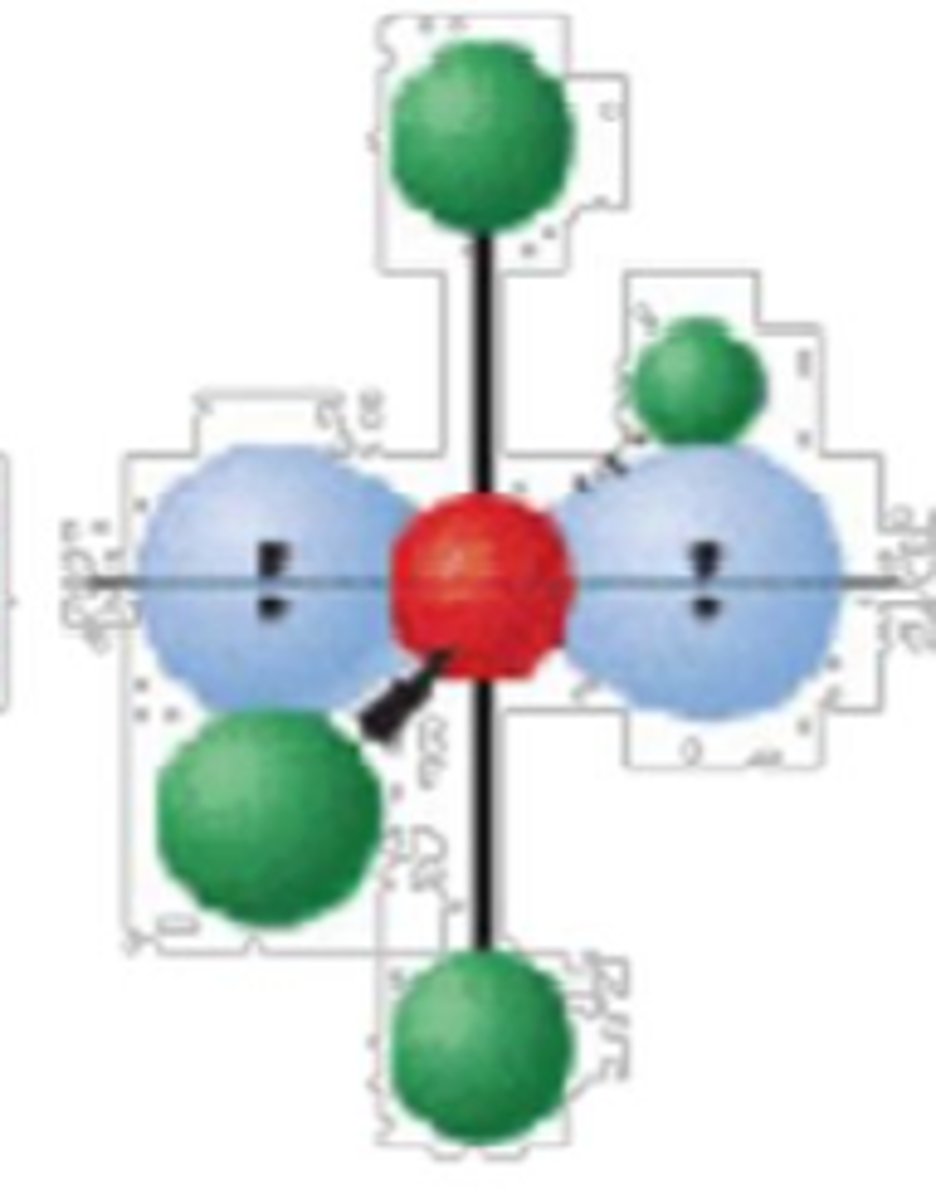
6 electron groups, 3 lone pairs
T-shape; <90°
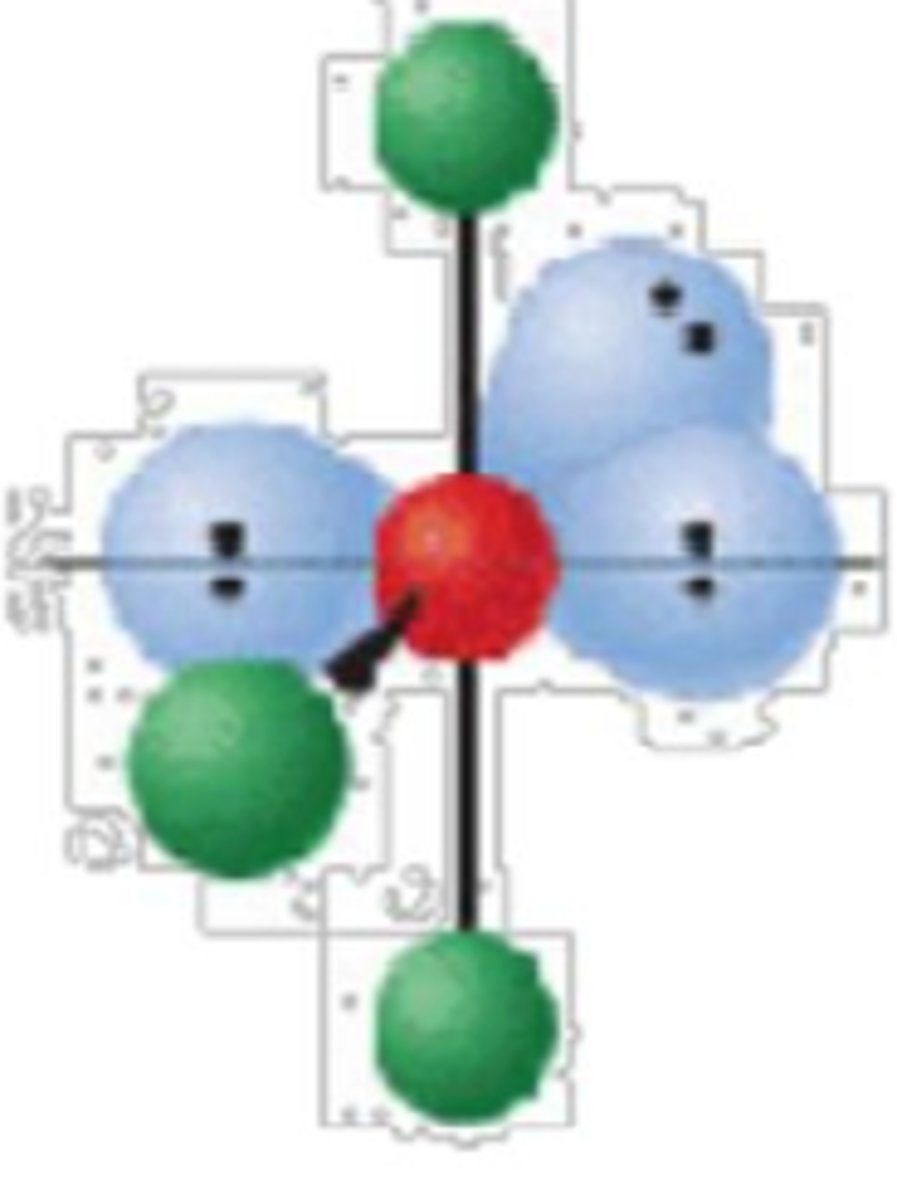
6 electron groups, 4 lone pairs
linear; 180°