Thermochemistry and Thermodynamics
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
state function
a function that only depends upon the initial and final states of the system, being independent of the pathway taken
zeroth law
concept of thermal equilibrium and temperature
conservation of energy
energy can not be destroyed or created, but converted from one form to another
spontaneous process
increase in entropy — G < 0, H < 0, S > 0
adiabatic
no heat in or out
isothermal
same temperature
isochoric
same volume
isobaric
same pressure
system does work on surroundings
-w
surroundings does work on system
+w
condution
heat transfer via molecular agitation within a material (but not involving net movement)o
convection
heat transfer due to the motion of a fluid (including gases)
radiation
heat transfer via electromagnetic radiation
bond breaking is ____
endothermic
bond making is ____
exothermic
spontaneous at all temperatures
H: -
S: +
-TS: -
nonspontaneous at all temperatures
H: +
S: -
-TS: +
spontaneous at low temperatures
H: -
S: -
-TS: +
spontaneous at high temperatures
H: +
S: +
-TS: -
solid to liquid
fusion
liquid to gas
vaporization
gas to solid
sublimation
liquid to solid
crystallization
gas to liquid
condensation
gas to solid
deposition
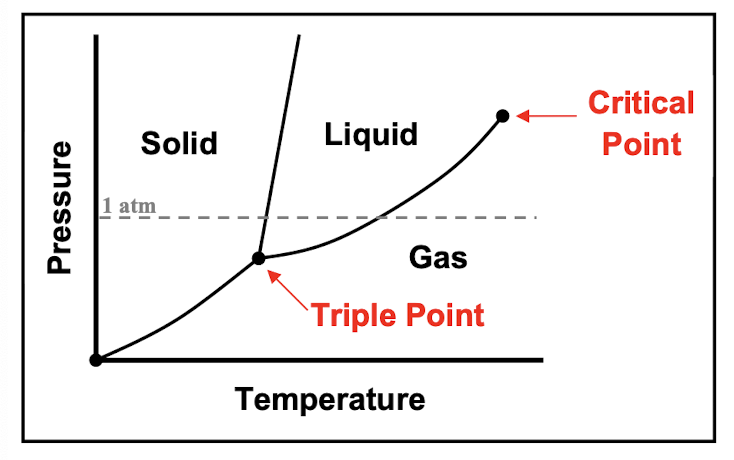
phase diagrams
triple point: the unique combination of temperature and pressure at which all three phases
critical point: the temperature and pressure where a substance can no longer exist as both a liquid and a gas, but instead as a supercritical fluid