Biology 101 - Chapter 3: The Solvent of Life
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Chemical Formula of Water
H2O, 2 hydrogen atoms, 1 Oxygen atom, has a polar covalent bond
Polar Covalent Bond
unequal sharing of e-, e- spends more time closer to O
Polar molecule
uneven charge distribution
Why is water the universal solvent?
Because it is a polar molecule.
What percentage of living organisms is made up of water?
70%-95%
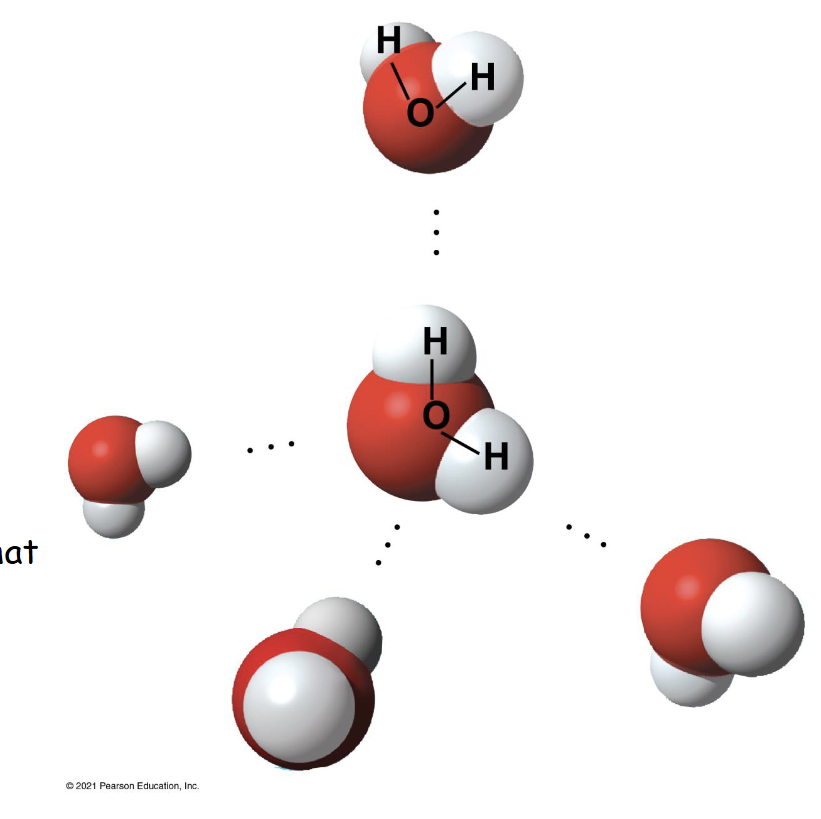
How many water molecules are pictured?
5
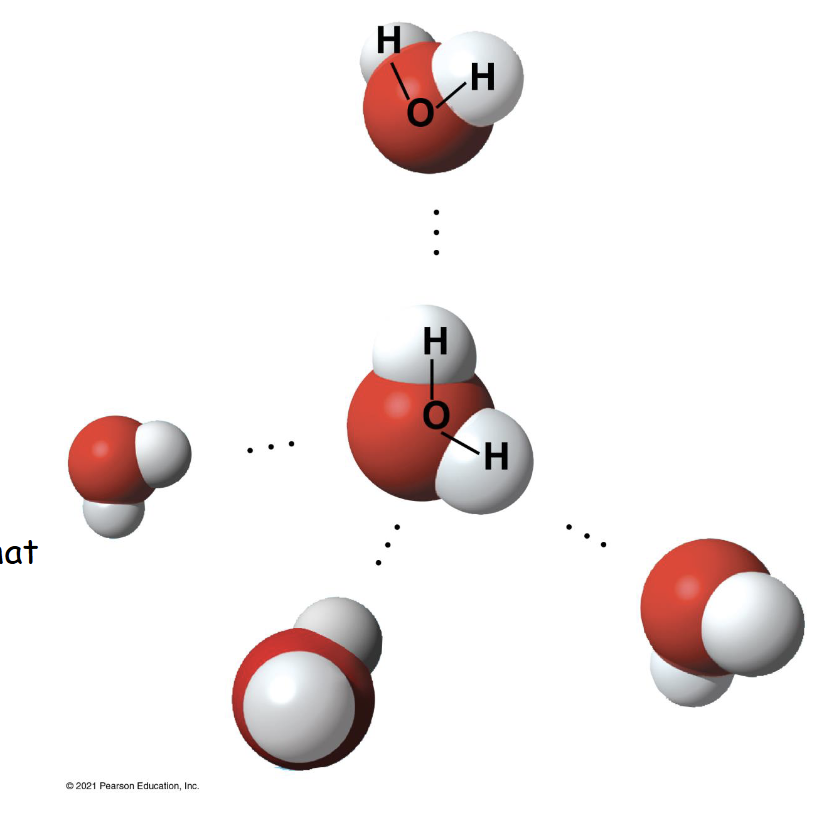
Where in this photo is a polar covalent bond?
Between hydrogen and oxygen
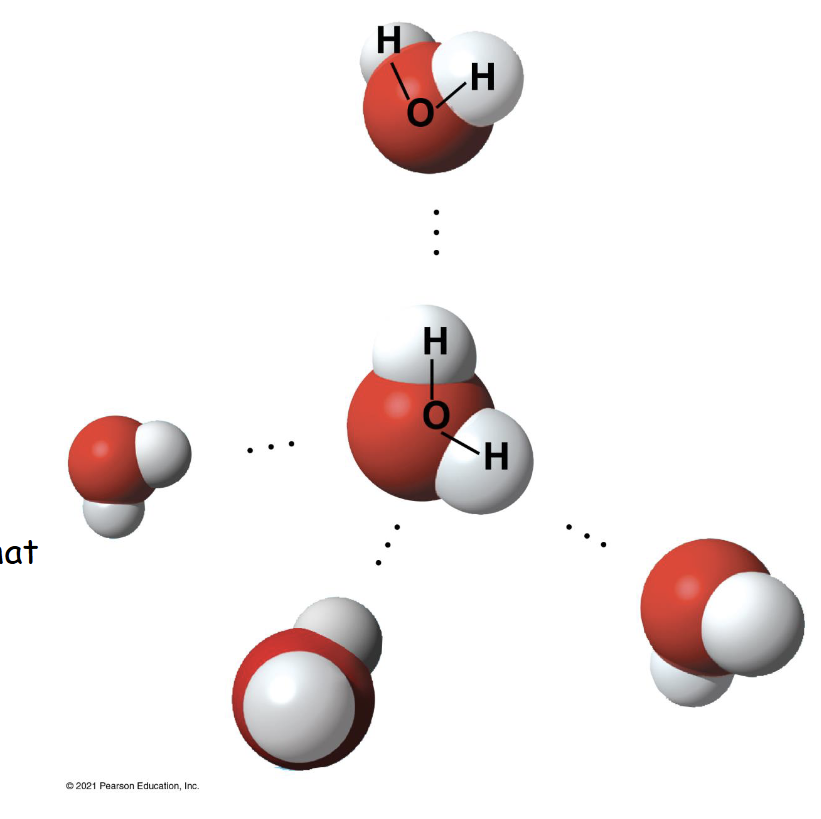
Where in this photo is a hydrogen bond?
Between the 8- charge and 8+ charge
What atom in a water molecule is more electronegative?
Oxygen
What are the 4 emergent properties of water?
cohesive behavior
moderation of temperature
ice floats on liquid water
water is the solvent of life
What type of bond holds water molecules together, creating cohesive behavior?
Hydrogen bonds
What is surface tension?
measure of how difficult it is to stretch or break surface of liquid.
How does water moderate temperature?
water absorbs heat and hydrogen bonds break
water releases heat and hydrogen bonds form
Why is it important that water moderates temperature?
creates an environment suitable for life
moderates coastal air temperatures