Orbitals and Electron Configuration (with rules and exceptions)
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
s orbital
spherically shaped
1 sub orbital
max 2 e-
p orbital
dumbbell shaped
3 sub orbitals
max 6 e-
d orbital
clover shaped
5 sub orbitals
max 10 e-
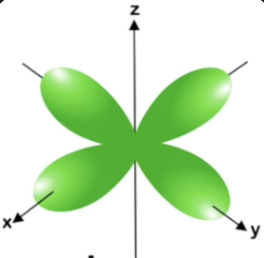
dx2-y2
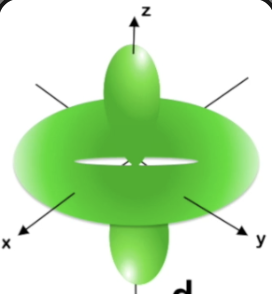
dz2
f orbital
7 sub orbitals
max 14 e-
d4 exceptions
Cr, Mo
d4 exceptions’ configuration
s1d5
d9 exceptions
Cu, Ag, Au, Rg
d9 exceptions’ configuration
s1d10
Tc config
[Kr] 5s14d6
Ru config
[Kr] 5s14d7
Rh config
[Kr] 5s14d8
Pd config
[Kr] 4d10
Pt config
[Xe] 6s15d9
Electron configurations for anions
add electrons in the same order as usual
Electron configurations for cations in the first two groups
Remove last electron added
Electron configurations for cations of transition metals
remove s sub orbital’s electrons first, then d sub orbital’s electrons
isoelectronic
an atom and ion are isoelectronic if they have the same electron configuration