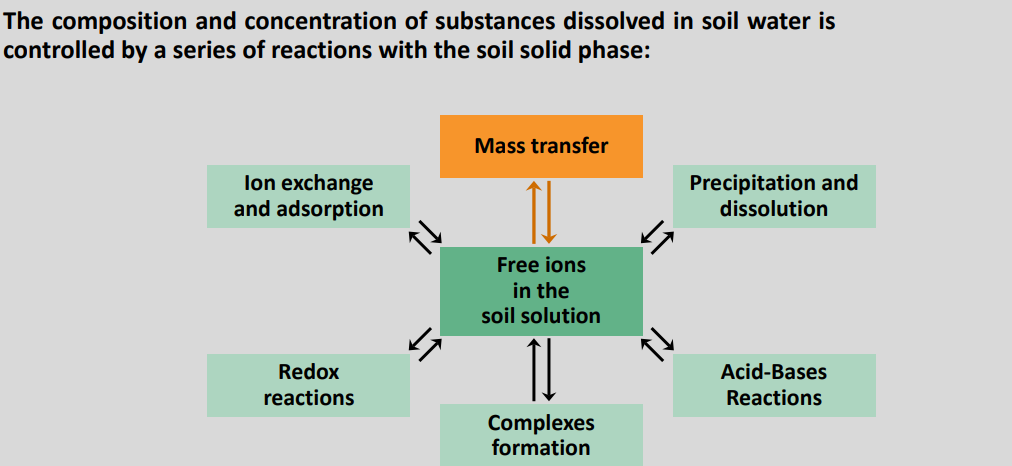
9-10 Interaction soil-liquid: soil solution
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
Interaction soil matrix - soil solution
Adsorption: Deposition (of ions, atoms, molecules) on surface (at interfaces)
Desorption: Redissolution and release into the mobile phase
Sorption: Besides surface adsorption, it also includes surface precipitation
Surfaces charges of soil component
The surface of almost all solid soil components is electrically charged, i.e. it attracts dissolved ions of opposite charge
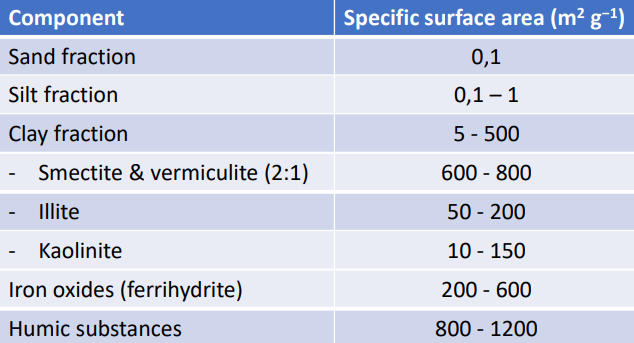
The most important charge carriers: fine components with a high specific surface area:
Clay minerals
Organic substance
Oxides and hydroxides (especially of iron, manganese, aluminium)
→ High environmental relevance
Specific surface area
Definition: the size of the surface area per unit mass of a solid substance, the unit is m2 g−1
Repetition of silicate
Flashcard – Main clay minerals (essential overview)
Kaolinite (1:1)
Structure: 1 tetrahedral + 1 octahedral layer
Interlayer: none (H-bonds)
Charge: ~0 (very low substitution)
Illite (2:1)
Structure: tetra–octa–tetra
Interlayer: K⁺ fixed
Charge: moderate (isomorphic substitution)
Smectite (2:1)
Structure: tetra–octa–tetra
Interlayer: exchangeable cations + H₂O
Charge: high (isomorphic substitution)
Clay minerals
Permanent negative charge
→ Caused by isomorphic replacement in the clay lattice (e.g. Si⁴⁺ → Al³⁺, Al³⁺ → Mg²⁺)
→ Typical of 2:1 clay minerals
→ Independent of pHVariable (pH-dependent) charge
→ From surface hydroxyl groups (≡Si–OH, ≡Al–OH)
→ Can be positive or negative depending on pHCation binding
→ Cations (K⁺, Na⁺, Ca²⁺, Mg²⁺) are heldon external surfaces
in interlayer spaces (especially in 2:1 clays)
Key consequence
→ High cation exchange capacity (CEC) and strong control on soil fertility
Acidic → mostly positive charges
Alkaline → mostly negative charges
Oxide
Surface groups: O⁻ and OH⁻ on oxide surfaces
→ Similar to edge sites of clay mineralsCharge mechanism: protonation / deprotonation
Fe–OH + H⁺ ⇌ Fe–OH₂⁺ (positive)
Fe–OH ⇌ Fe–O⁻ + H⁺ (negative)
pH effect:
↑ pH (↓ H⁺) → less positive, more negative charge
↓ pH → more positive charge
Key property:
→ Only variable charge (no permanent charge)
→ Charge is fully pH-dependent
Organic matter
Main functional groups:
Carboxyl (–COOH)
Phenolic & alcoholic –OH
Charge mechanism:
Dissociation of H⁺:
–COOH ⇌ –COO⁻ + H⁺↑ pH → ↑ dissociation → more negative charge
Key property:
→ Only variable charge
→ Charge is pH-dependent (no permanent charge)Importance:
→ High contribution to CEC, nutrient retention, and aggregation
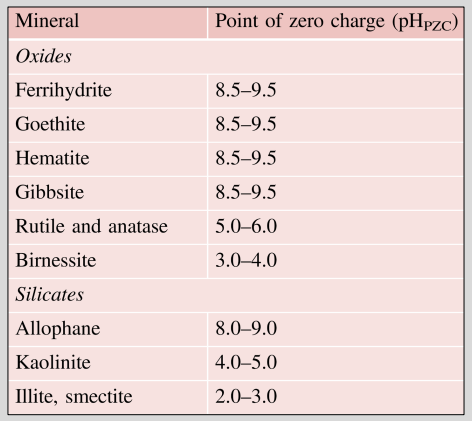
Point of zero charge
Definition: pH value at which a surface carries an equal amount of positive and negative charge, noted pHPZC
→ a net charge of zero
→pH below pHPZC means mostly positive charges
→pH above pHPZC means mostly negative charges
Variable charges (pH dependent charges)
What it is: Charge on soil particles that changes with pH
Mechanism: Protonation / deprotonation of surface –OH groups (oxides, OM, clay edges)
pH effect:
Low pH → more positive charge
High pH → more negative charge
Materials with variable charge:
Oxides (e.g. goethite)
Kaolinite (low permanent charge)
Organic matter
Compared to 2:1 clays:
Smectite, illite → mainly permanent negative charge (less pH-dependent)
In most soils: negative charges dominate
Key concept: pHₚzc (point of zero charge) = pH where net charge = 0
surface charges of soil component (summary)
Permanent charge:
Clay minerals (2:1 clays) → permanent negative charge from isomorphic replacement (independent of pH)
pH-dependent (variable) charges:
Clay edges & fracture surfaces → gain or lose H⁺ depending on pH
Soil organic matter (SOM) → mainly negative charge from dissociation of carboxyl (–COOH) and phenolic –OH groups (↑ with pH)
Fe and Al oxides → can be positive or negative, controlled by pH (surface –OH groups)
Key idea:
Total soil charge = permanent clay charge + variable pH-dependent charge
In most soils, negative charges dominate
Absorption mechanism
Adsorption Isotherms
Describe adsorption of substances in soils: Relationship between adsorbed quantity and equilibrium concentration in the solution at constant temperature
Ion Exchange between Soil Particles, Soil Solution & Plant Roots
Nutrients are found in two elements in the soil:
soil particles → where most of them are found
soil solution → dissolved ions, few in number, it’s only a means of transport
→ Plants can only take up nutrients from the soil solution, not directly from solid particles.
Particles have more nutrient because:
Clay minerals and organic matter have negative charges
Some oxides can have positive charges
Opposite charges attract → ions are adsorbed on surfaces
→ not permanent
Ion exchange = swapping ions without changing total charge
Example (cation exchange):
A soil particle holds Ca²⁺
The soil solution brings 2 NH₄⁺ or 2 H⁺
They swap places because charges must stay balanced
So:
Ca²⁺ is released into soil water
NH₄⁺ (or H⁺) takes its place on the surface
→ Nothing is destroyed, nothing is created — only exchanged
Plant roots
They:
Release H⁺ (protons) into the soil
This acidifies the root zone slightly
What happens then:
H⁺ competes for negatively charged sites
Nutrient cations (Ca²⁺, K⁺, Mg²⁺, NH₄⁺) are pushed off the soil particles
These nutrients enter the soil solution
Roots absorb them immediately
→ Roots “unlock” nutrients from soil particles
Important because:
1. Continuous nutrient supply
Even if soil water contains little nutrient, exchange keeps replenishing it.
2. Protection against leaching
If all nutrients were dissolved → rain would wash them away
Adsorption acts like a buffer
3. Nutrient storage
Soil works like a warehouse, not a pipeline.
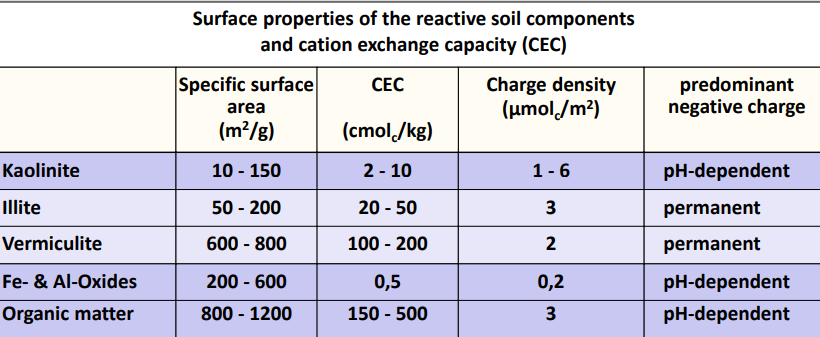
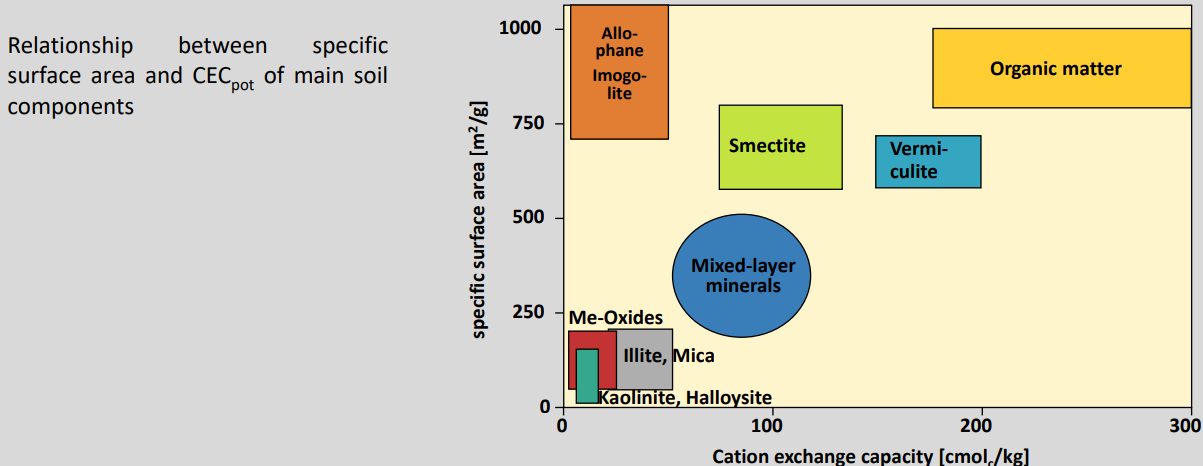
Cation exchange capacity (CEC) of main soil component
Cation exchange capacity (CEC) = maximum adsorbable cation quantity per mass of soil (cmolc/kg)
CEC of soils
The CEC of soils fluctuates in a wide range, depending on:
Texture
Type of clay minerals
SOM content
Common values are between 5 and 100 cmolc/kg
Effective CEC (CECeff) at the current pH value of the soil
Potential CEC (CECpot) with a reference pH of 7
CEC and pH
Core idea:
Cation Exchange Capacity (CEC) increases with pH, mainly due to pH-dependent (variable) charges.
Key points:
Permanent charge (from clay minerals) is constant, independent of pH.
Variable charge (from organic matter and oxide/clay edges) increases as pH rises (deprotonation).
Therefore:
Acidic soils: CECeff<CECpot → many charges not yet expressed
Neutral soils: CECeff = CECpot
Main contributor to pH effect:
Organic matter contributes more to pH-dependent CEC than the mineral fraction, especially in topsoils.
Bottom line:
Higher pH → more negative charges → higher effective CEC → better nutrient retention.
Base saturation
Definition:
Base saturation (BS) is the percentage of the CEC occupied by base cations.
Base cations:
Ca²⁺, Mg²⁺, K⁺, Na⁺
(H⁺ and Al³⁺ are acid cations)
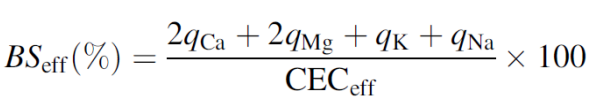
Interpretation:
High BS → many nutrient cations, higher fertility, higher pH
Low BS → dominance of H⁺/Al³⁺, acidic soil
Key idea:
BS links CEC, soil acidity, and nutrient availability.
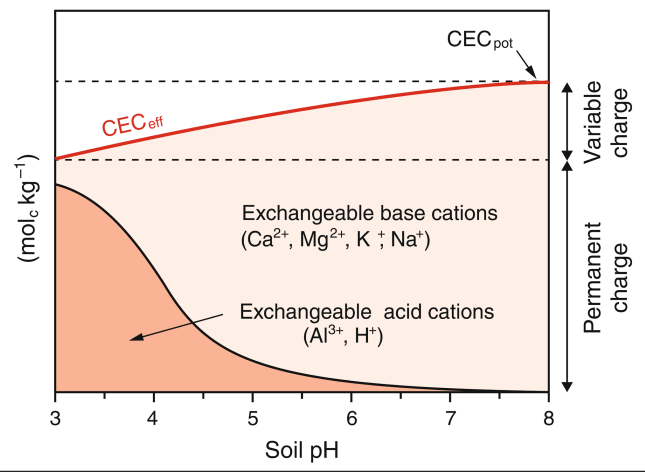
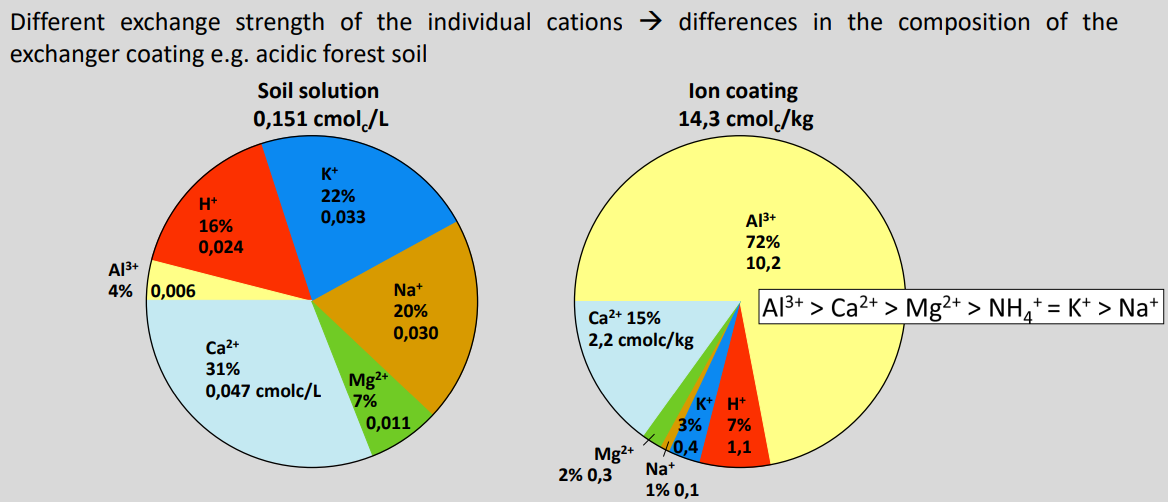
Cationic coating of a soil
As soil pH decreases, the effective CEC (CECₑff) decreases and soil surfaces become increasingly coated with acid cations.
Mechanism:
Lower pH → fewer negative variable charges on clays, oxides, and organic matter
→ CECₑff decreases (potential charge not fully expressed)
Affinity of cations to negative charges
What controls affinity?
Cation charge
Higher charge → stronger attraction (Al³⁺ > Mg²⁺ > Na⁺)
Hydration shell
Smaller hydrated radius → stronger binding
Cations must shed part of their hydration shell to bind
Strongly hydrated ions bind less easily (e.g. Na⁺)
Solution concentration
Higher external concentration → higher adsorption
Affinity series (strong → weak):
Al³⁺ > Ca²⁺ > Mg²⁺ > NH₄⁺ ≈ K⁺ > Na⁺
Key idea:
Valence + hydration control how tightly cations bind to negatively charged soil surfaces.
Cation distribution between soil solution and ion coating
Anion exchange
Important anions in soil
Cl-
NO3 -
SO4 2-
PO4 3-
Organic anions and dissolved organic matter (DOM, mostly acids)
Factors influencing anions binding
Type and charge of anion
Concentration of the anion in the soil solution
Composition of the adsorbents
pH value (more acidic, more AEC)
→ Similar to CEC but in reverse!
1) Different anions can compete with each other. Example: PO4 3- and AsO4 3-
2) Adsorbed amount increases with increasing concentration in the solution (like cations)
3) Adsorbents: clay minerals and especially Al, Fe hydroxides and oxides, allophane; variable charge only
4) Strong pH influence: Increase of adsorption with decreasing pH (variable charge)
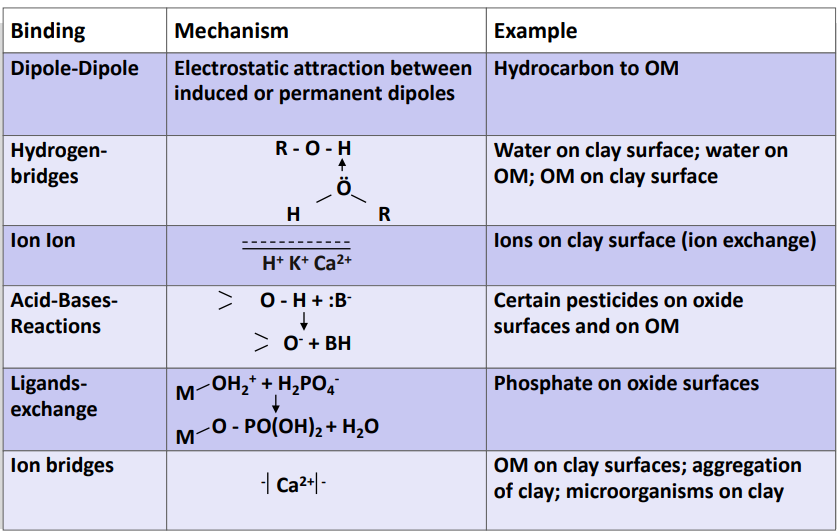
Adsorption mechanism: outer sphere
What it is: Weak, purely electrostatic binding (and/or H-bonds) between ions and charged soil surfaces
No direct chemical bond to the surface → no ligand exchange
Hydration shell remains intact (water layer stays between ion and surface)
Occurs in the electric double layer
Reversible ion exchange (e.g. Cl⁻ ⇄ NO₃⁻)
Also called physisorption
Strength depends strongly on ionic strength of the soil solution (generally weak)
→ Typical for non-specific adsorption of ions on oxide and clay surfaces
Adsorption mechanism: inner sphere
True chemical bonding via ligand exchange with (hydr)oxides
Hydration shell partly/fully lost
Strong and specific
Little dependence on ionic strength
Typical ions: PO₄³⁻, AsO₄³⁻, Cu²⁺, Zn²⁺
Can be mono-/bidentate, mono-/binuclear
= Chemisorption
Main differences between outer and inner
Outer-sphere = weak & electrostatic
Inner-sphere = strong & chemical
pH of the soil
pH values reflects:
Soil development and resulting soil chemical properties
Behaviour of nutrients and pollutants
Suitability of soil for plant growth, habitat for soil MO, filtering pollutants
→Most important and most meaningful soil parameter that can easily be measured (together with texture and OM content)
Soil become more acidic with development in humid climate
More H+ added (rain, soil processes) than can be neutralised by the soil
Soluble product of chemical reaction with H+ are soluble and washed out: Loss of buffer function
Important to understand what is going on in the soil
Soil acidity
based on soil content:
Exchangeable/dissociable H+ ions
Exchangeable Al3+ ions
Al3+ is present in the soil solution in hydrated form and can dissociate H+. Simplified equation:
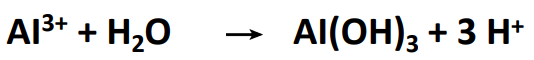
Al ions only occur in the soil solution from pH < 5
Soil pH typically ranges ~3–8 (most soils 5–6.5).
Lower pH → higher Al³⁺ in soil solution → Al toxicity to roots.
pH is measured in H₂O (actual pH) or CaCl₂/KCl (more reproducible); usually pH(CaCl₂) ≤ pH(H₂O).
Proton sources in the soil → carbonic acid
Source: Root and microbial respiration
Key idea: CO₂ produced in soil forms carbonic acid → releases H⁺
Importance: Main natural acidification process in soils (pH ~7–5), continuous/inexhaustible
Proton sources in the soil → Organic acids
Source: Roots and organic matter decomposition
Key idea: Dissociation of organic acids releases H⁺
Role: Mobilises nutrients (e.g. P, metals) and contributes to soil acidity
Proton sources in the soil → cation uptake by plants
Source: Plant nutrient uptake
Key idea: Uptake of base cations (K⁺, Ca²⁺, Mg²⁺) is balanced by H⁺ release
Effect: Rhizosphere becomes more acidic
Note: Acid balance restored only if biomass is not harvested
Proton sources in the soil → Nitrogen transformations (nitrification)
Source: Microbial N cycling
Key idea: Conversion of NH₄⁺ to NO₃⁻ releases H⁺
Importance: Major acidification process in fertilised and forest soils
Proton sources in the soil → Oxidation of Fe²⁺ and sulfides
Source: Oxidation of Fe²⁺, Mn²⁺, and sulfide minerals
Key idea: Oxidation reactions release H⁺ (or sulfuric acid)
Effect: Strong acidification, typical in acid sulfate soils
Proton sources in the soil → acid rains
Source: Atmospheric SO₂ and NOₓ deposition
Key idea: Acidic inputs from the atmosphere add H⁺ to soils
Impact: Contributes to long-term soil acidification, especially in sensitive soils
Proton sources in the soil → Loss of Acid Neutralization Capacity (ANC)
Source: Combined biological and chemical processes
Key idea: Removal of base cations and proton inputs reduce buffering capacity
Result: Soil becomes more vulnerable to acidification over time
Soil changes due to acidification
1. Decline in cation exchange capacity
2. Decline in nutrient availability
3. Reduction in biological activity
4. Increase in heavy metal mobility
5. Increase of Al concentration in soil solution and water bodies and thus Al toxicity to plants
Tropical and ancient soils are very acid
Also places where you have lot's of industrial production really acid (USA…)
Soil as a buffer
Buffering: reversible or irreversible binding of H+ ions
Stabilization of the pH value (= buffering)
pH value changes only when buffer substance is used up
pH ranges, in which substances buffer, overlap
→ Various buffersystems are active in the soil
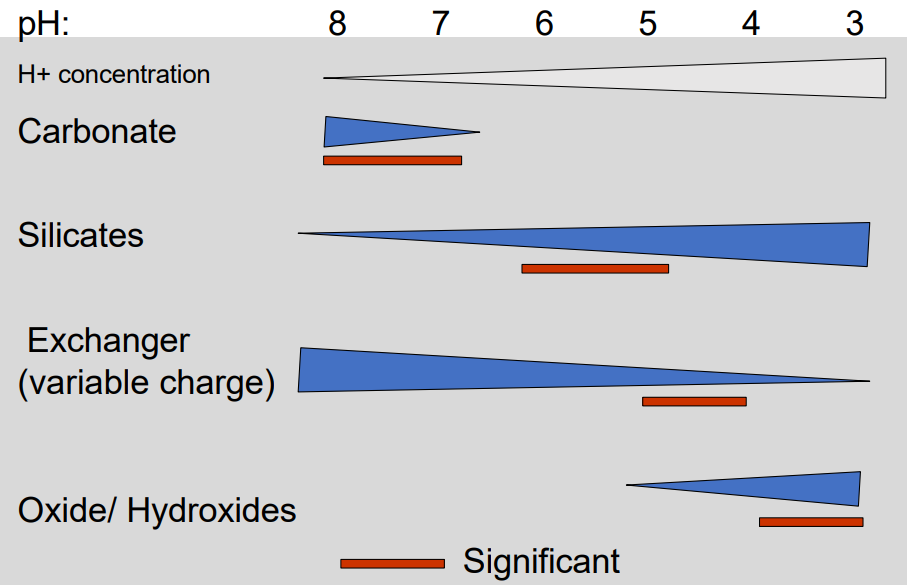
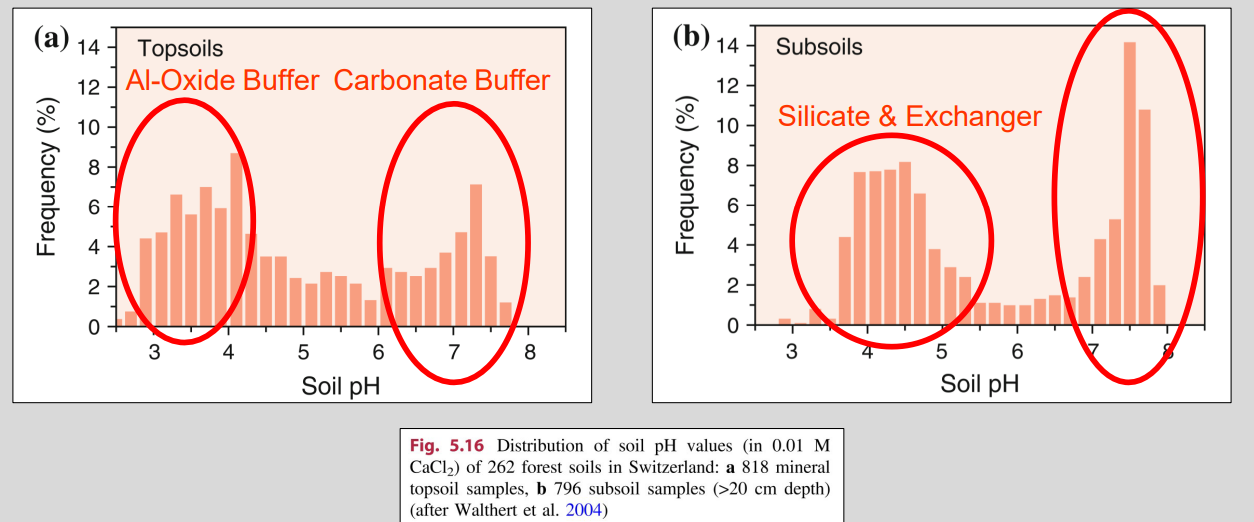
Carbonate buffer
Where: Carbonate-rich soils (CaCO₃ present)
Key idea: Carbonates neutralize incoming acidity by consuming H⁺
Effect on pH: Soil pH is strongly buffered and stays ≥ ~7
Buffer range: ~6.5 – 8
Limit: Dissolved Ca and bicarbonate can be leached → buffer weakens over time
Silicate buffer
What it is:
pH buffering by silicate minerals (especially clays) via surface silanol groups (Si–OH).
How it works:
H⁺ is consumed by silicate surfaces
Adsorbed K⁺ and Al³⁺ are released into solution
Effective pH range:
~4.2 – 6
Key consequence:
Continued acidification → silicate breakdown
Released Al³⁺ can occupy clay interlayers or exchange sites
Role:
Important buffer after carbonates are depleted, but weaker than carbonate buffer.
Al & Fe Oxide Buffer
Mechanism:
Protonation and dissolution of Al/Fe (hydr)oxides consume H⁺.
pH range:
Al hydroxides: ~pH 3–4.8
Fe oxides: < 3
Key effect:
Strong buffering at very low pH, but releases Al³⁺ / Fe³⁺ → toxicity risk.
Variable charges buffer
Where:
Organic matter, clay minerals, Fe/Al (hydr)oxides
Mechanism:
H⁺ binds to variable surface charges → base cations (Ca²⁺, Mg²⁺, K⁺) are released.
pH range:
~ 3–10 (material-dependent)
Key effect:
Buffers acidity but promotes base cation leaching → soil impoverishment over time.
Buffer system
Importance of redox reactions
Colour
Mobility of metals
Toxicity of trace elements
C, N, S, Fe, Mn cycles
Nutrient availability (e.g. P)
pH value
Oxidation processes produce acid (H+)
Reduction processes are acid buffers
Organisms are involved in redox reactions
Redox reactions
Oxidation = loss of electrons
Reduction = gain of electrons
Electron donor = reducing agent (is oxidized)
Electron acceptor = oxidizing agent (is reduced)
In soils: organic matter is the main electron donor
Most soil redox reactions are microbially driven (e.g. oxidation of organic C coupled to O₂ reduction)
Redox potential
Redox potential (E or E°) measures how strongly a substance tends to gain or lose electrons (expressed in V or mV).
More positive E° → substance easily gains electrons → strong oxidizing agent → undergoes reduction.
More negative E° → substance easily loses electrons → strong reducing agent → undergoes oxidation.
Direction of electron transfer: electrons flow from lower E° to higher E°.
A redox reaction always couples two half-reactions (one oxidation, one reduction).
Overall reaction potential: E°cell = E°reduction - E°oxidation
In soils, redox potential reflects oxidation–reduction conditions (e.g. well-aerated = high E, waterlogged = low E).
pH dependence of redox potential
In soils, redox reactions often involve H⁺, so they are pH-dependent.
Reduction reactions usually consume protons (H⁺) → tend to increase pH.
Oxidation reactions usually release protons (H⁺) → cause acidification.
Therefore, redox state and pH are tightly coupled in soils.
Chemical weathering: oxidation
Oxidation of Fe²⁺, Mn²⁺ and sulfides (S²⁻) during weathering.
Produces Fe³⁺, Mn³⁺/Mn⁴⁺ and sulfate (SO₄²⁻).
Releases H⁺ → acidification of soils and waters.
Key example: pyrite oxidation, responsible for acid mine drainage.
Redox reactions in soil
Core idea:
Soil redox conditions depend on oxygen availability and control which electron acceptors microbes use.
Key points:
Organic matter = main electron donor
Aerobic soils: O₂ is used → highest redox potential (Eh)
Flooded / waterlogged soils: O₂ is consumed → soil becomes anaerobic → Eh decreases
Microbes then use electron acceptors in a fixed sequence as Eh drops:
O₂ → NO₃⁻ → Mn oxides → Fe oxides → SO₄²⁻ → CO₂As long as one acceptor is present, it buffers Eh; Eh drops further only when it is depleted
Consequences:
Appearance of Mn²⁺, Fe²⁺, sulfide, CH₄ at low Eh
Strong effects on nutrient availability, metal mobility, and toxicity
Key point:
When oxygen is depleted, soils become anaerobic and microbes switch step-by-step to weaker electron acceptors, causing a progressive decrease in redox potential (Eh).
Morphological characteristics in soil profile
Reducing conditions (waterlogged, low O₂):
Fe and Mn oxides are reduced and dissolve
Fe²⁺ and Mn²⁺ become mobile
Soil colors: grey / blue-green, bleaching
Typical of hydromorphic (gley) soils
Oxidizing conditions (well-aerated):
Fe²⁺ and Mn²⁺ are oxidized and precipitate
Formation of Fe(III) and Mn(IV) oxides
Soil colors: rusty red (Fe), black concretions (Mn)
Key idea:
Water saturation controls redox state → reduction = dissolution & bleaching, oxidation = precipitation & staining.
redox summary
Redox reactions = oxidation + reduction (electron transfer)
They affect soil pH:
Oxidation → acid production
Reduction → acid buffering
As redox potential drops, electron acceptors are used sequentially (O₂ → NO₃⁻ → Mn → Fe → SO₄²⁻ → CO₂)
Energy yield decreases along this sequence
Involve key element cycles: C, N, S, Fe, Mn
Visible in soil profiles by characteristic color patterns (red, black, grey/blue-green)
Key idea: Redox reactions control soil chemistry, pH, energy use, and soil colors.