Chemistry 102 - Exam 2 materials
1/100
Earn XP
Description and Tags
Lecture 11-17
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
101 Terms
What are intermolecular forces?
The attraction BETWEEN neighboring molecules in the liquid and solid phase (like H2O to H20). It includes London dispersion, hydrogen bonding, dipole-dipole,and ionic force
What are intramolecular forces?
Bonding WITHIN a molecule that holds atoms in a compound together. It includes covalent and ionic BONDS
What level of intermolecular forces do gas, liquids, and solids have? which ones are more tightly bonded?
Gas - no IMF
Liquid - Intermediate
Solid - Strong (tightly bonded)
How do you phase change in the context of IMF’s?
You break or form IMF
What is the relationship between strong IMF’s and boiling, melting, freezing, and fusion point?
Directly proportional
What is normal boiling point?
The temperature (at 1atm) where a liquid becomes a gas
What is normal freezing point?
The temperature (at 1atm) where a liquid becomes a solid
What is the molar heat of vaporization?
The amount of energy required to boil one mole of a liquid to 1 mol of a gas
For H20 (l) to H2O (g) - ΔHvap = 40.6kj/mol
What is the molar heat of fusion? Why is the ΔHvap smaller than that of the molar heat of vaporization?
The amount of energy required to melt one mole of a solid (to one mol of a liquid)
For H20 (s) to H2O (L) - ΔHvap = 6.02 kj/mol
It is small because this phase change only breaks 15%
What percentage of breaking is required to go from a solid to a liquid to a gas?
solid —- 15% —→ liquid —— 85% —→ gas
What is the relationship between IMF’s and vapor pressure?
inversly proportional
What is vapor pressure?
A pressure (force) exerted by vapor. In this, gas moves more dynamically (collide with wall more frequently) but there are weak bonds between them. High temp, high vapor pressure, low intermolecular forces
Describe how vapor pressure works
Certain molecules can gain enough energy to break through the IMF’s on the surface of the water that held them in the liquid phase (also known as the surface phenomenon - liquid to gas).
What is equilibrium for vapor pressure?
When the rate of two opposing processes are exactly equal (for vapor is when the energy for liquid to become a gas = the gas to become a liquid) the amount becomes constant and the pressure of the vapor is VAPOR PRESSURE
What is the relationship between vapor pressure and temperature?
Directly proportional
What are ionic forces? Are they strong?
A type of intermolecular force that is electrostatic between cations and anions
Ionic forces are always much stronger than the IM forces that hold covalent compounds together. Because of this, ionic compounds have high melting and boiling points, with low vapor pressure
Define electrostatic
attraction or repulsion force between charged particles. extremely strong
What are covalent forces?
IMF’s that hold covalent compounds together in the solid and liquid phases (dipole-dipole, hydrogen, and londan dispersion).
What are the types of covalent forces?
Dipole-dipole
Hydrogen bonding (H-bonding)
London dispersion (LD forces)
What are dipole-dipole forces?
Holds polar covalent (different electronegativity) compounds together (solid and liquid phases). Only compounds with a dipole moment (polar compounds) exhibit dipole-dipole forces (makes a partial + and partial -).
How can you use dipoles to see if a molecule is polar?
If they cancel out they are non-polar (look at electronegativity to drive dipoles)
Is the relationship beyween C-H polar?
No its non-polar because the electronegativity difference is so small
What is hydrogen bonding?
A special type of dipole-dipole force
It is the strongest covalent IM force
Any covalent H-bonding has an irregularly high melting or boiling point
Must have H covalently bonded to one of the three (F, O, N) most electronegative elements in the periodic table to form H bonding. Must also have H-F, H-N, and H-O (with a lone pair) to be considered irregular
Its strong because H has low EN so very polar and H is tiny which maximizes attraction
What is the general relationship between molar mass and boiling point?
directly proportional
Why is H-O more polar and stronger than H-F?
The lone pair phenomenon allows neighboring molecules to interact with other molecules. H2O allows for more bonding (two other molecules).
What are london dispersion forces?
All covalent molecules have these due to accidental dipoles
Electrons can be pushed to any side of the atom and creates an “accidental” dipole which creates one in the neighboring molecule
This is the ONLY type of intermolecular force that NONPOLAR compounds exhibit
Size and shape are two factors that affect the strength of LD. In general, larger covalent compounds have stronger LD forces. The more elongated the molecule, the stronger the LDF
What is the relationship between elongation of a molecule and strength of a bond? Why?
The more elongated the molecule, the stronger IMF’s. This is because of surface area.
For questions where you have to order IMF’s or vaporization or boiling point, what are the steps?
Identify the IMF’s
If they’re LD, go based on mass
Order the types of bonds based on strength?
Ionic
Hydrogen
Dipole - Dipole
London
What is the electronegativity exception with H?
It is between B and C and the same as P
How do you determine if boiling point is the same between two molecules?
Molar mass (Or IMF’s)
What are condensed states?
Only liquids and solids
During physical changes, do the molecules stay intact?
Yes, only the forces between them change
What is condensation?
When vapor molecules reform a liquid
What is a heating curve?
A plot of temperature versus time for a substance where energy is added at a constant rate
Are physical changes endothermic or exothermic?
Solid to liquid to gas is endothermic
Gas to liquid to solid is exothermic
If you are comparing the strength between two only London dispersion forces, how do you know which is stronger?
The one that is larger is stronger
What does 1 mole represent?
6.022 times 10 ^ 23 things
What are molecules?
The formula gives the number of atoms in a compound (H2O - 2 H 1 O)
How can you go from moles to molecules to atoms
Moles → Molecules (multiple the number by avogadros number)
Molecules → Atoms (multiply by how many atoms per molecule)
What is molar mass?
The mass of all the parts in one mole of a substance (add up all g/mol)
What is the molecular formula?
The empirical formula times N
What is the empirical formula?
The smallest whole number ratio
How do you find mass percent?
Mass of X/Total mass (times 100)
How can you determine the empirical formula?
Divide by the smallest mole
How can you find molecular formula?
Divide true molar mass by empirical molar mass
What are chemical reactions?
Reorganization of reactant molecules to form product molecules (break/make chemical bonds)
Atoms are conserved
gas, liquid, solid, aquous (reaction is run in H20)
What are the diatomics?
HONCLBRIF, written with 2 subscript
What are the six types of chemical reactions?
Synthesis: 2 or more reactants produce 1 product
Decomposition: 1 reactant produces 2 or more products
Single-displacement: An element replaces another in a compound
Double-displacement: Two ions exchange partners
Combustion: __ + O2 makes CO2 + H20
Redox: Electrons are transferred. Many of the above reactions can also be classified as redox
Balanced chemical equations imply what three things?
Number of atoms are conserved
Mass is conserved
Relates the mole ratios
What is a “perfect reaction”?
One in which both reactants are limiting/run out at the same time
What are limiting reactants?
Run out at the same time, determines how much product is made
What does the coefficient of a balanced chemical reaction represent?
The number of molecules/moles
What is the theoretical yield for a reaction, and how does this quantity depend on the limiting reactant?
The theoretical yield is the stoichiometric amount of product that should form if the limiting reactant is completely consumed and the reaction has 100% yield.
What are the two most common types of double displacement reactions?
Precipitation - a solid precipitate is formed
Acid/base (proton transfer reaction) - produces water and a salt (ionic compound)
What is a solution?
Homeogenous mixtures composed of a solvent and a solute (doesn’t need to be liquid)
What is a solvent?
Dissolving medium
What is a solute?
Substance dissolved (present in smaller amounts)
What is the main equation of a acid/base reaction?
H+ + OH- = H20
What does “like dissolves like” mean?
H20 is a polar solvent because it has polar bonds/dipole moment. Polar solvents dissolve polar-covalent compounds (and sometimes ionic)
What is hydration?
When “aq” is after a substance, its soluble in water. Occurs because partial +’s attract positive -’s
How are solutes classified?
By their ability to conduct electricity. Must be soluble to be classified
Strong (many ions), weak (few ions), or non-electrolytes (none)
What is the actual process of classifying a solute?
Is it ionic or covalent?
If ionic→ Is it soluble? Yes = strong. No= no designation
If covalent→ Is it an acid? Yes= is it strong. Not a strong acic = weak. Not an acid = nonelectrolye.
Why are acids an exception to the covalent solutes?
Acids (H in front) have the ability to partially break apart (behave as weak electrolytes) or fully break apart (strong)
What are the strong acid electrolytes to memorize?
HCl
HBr
HI
HNO3
H2SO4
HClO4
Is NH3 a strong or weak electrolyte?
Weak electrolyte, it partially dissociates
How do you determine if a reaction occurs?
If one of the products is soluble
What three types of balanced chemical equations do we need to know? Describe them
Balanced Chemical Formula Equation: Full with only coefficients and (state)
Complete Ionic: Broken apart with coefficient, charge, and state
Net Ionic: Get rid of the spectator ions and only place the ones that contribute to the solid
What is an acid? What are its types?
Proton donors
Monoprotic
Diprotic
Triprotic
^ used to determine how many hydrogens are acidic and can be donated to a base (only how many are in front).
What are bases?
H+ acceptors. Take them from acid to form H2O
What are dissociation reactions?
compounds dissociate completely to give ions in a solution. If they dissociate a lot, theyre strong
What is molarity?
Moles of solute/liter of solute
What are dilutions? What is special about them in terms of molarity?
Dilutions occur when you add water to have a desired molarity. Moles of solute present don’t change when water is added
What is the ideal gas law equation? What are its components?
P (pressure in atm) V (Volume in L) = n (moles of gas) R (gas constant = 0.08206L*atm/mol*k) T (temperature in kelvin)
What are the 2 major unique properties of gas?
Gases are mostly empty space as compared to solid and liquid phases (easily compressible).
Exert uniform pressure on containers (due to constant random motion)
What is pressure?
Force of collison/area
What are some common conversions between torr and atm and mmHG
1 torr = 1mmHg
1atm = 760 torr = 760mmHg
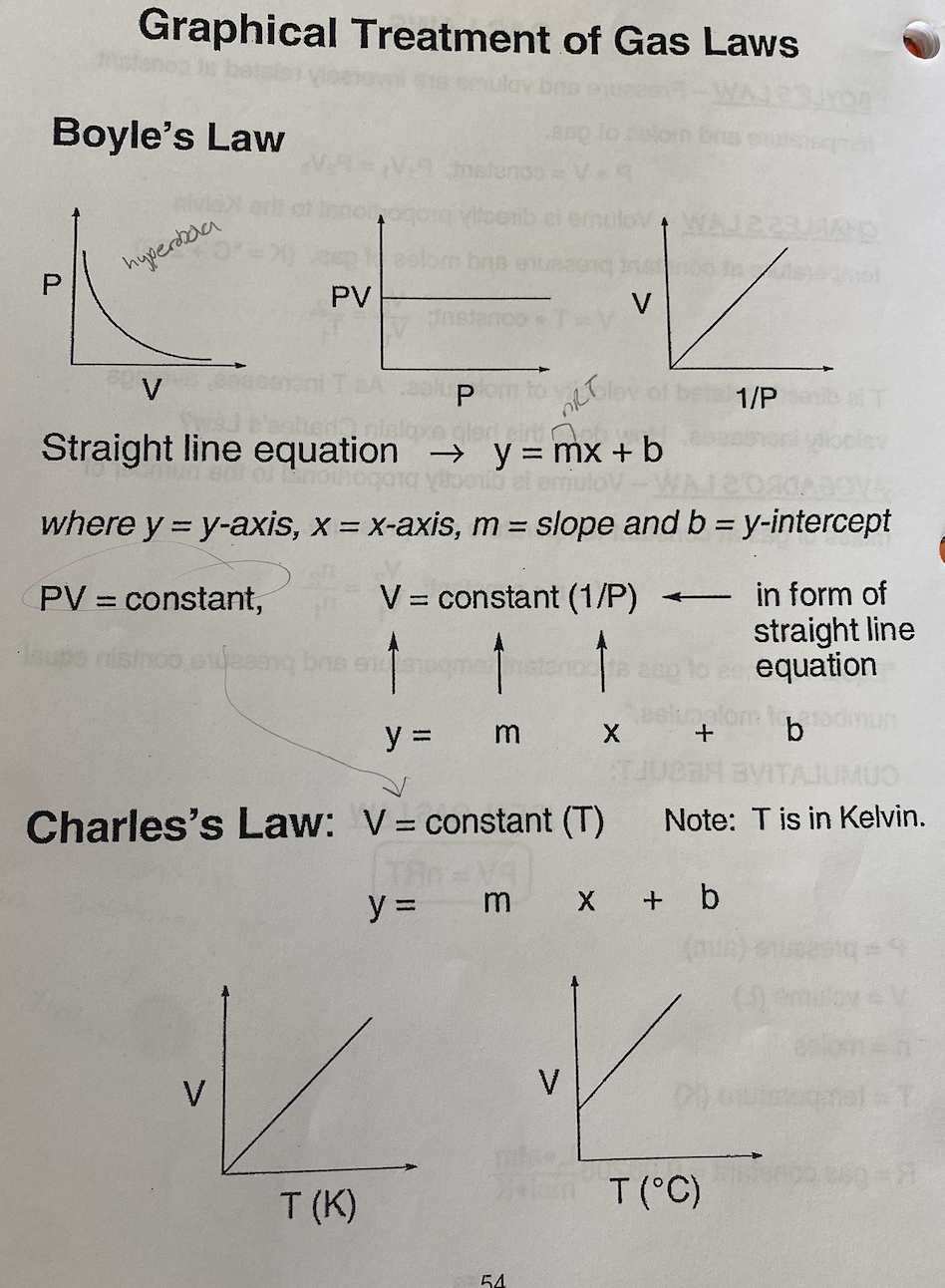
What is Boyle’s Law?
Pressure and volume are inversely related at a constant temperature and moles of gas (P1V1=P2V2). This is because constant moles become squished, so more increased collisions per unit area changes pressure.
What is Charles Law?
Volume is directly proportional to the Kelvin temperature at constant pressure and moles of gas (V2/V1=T2/T1). This is because a flexible container must have a matched pressure on inside to outside.
What is Avogadros Law?
Volume is directly proportional to the number of moles of gas at constant temperature and pressure (V2/V1=N2/N1). This is because the inside must equal the outside, and because equal volumes of gases at constant T and P contain equal #’s of moles.
What is the conversion from degrees celsius to kelvin
degrees celcius + 273
What is the difference between a rigid container and a flexible container
Rigid container = constant volume
Flexible = Constant pressure
What is Kinetic Molecular Theory? What are the five postulates?
A model that attempts to explain ideal gas behavior:
Gases are mostly empty space; the volume of the particles in negligible
Gas particles are in constant random motion. Pressure is due to the collisions of the gas particles within the container walls
Gas particles neither attract nor repel each either (since they’re far apart)
Pressure is due to collisions of gas particles with container walls (P=F/A)
The average kinetic energy of a gas sample is proportional to the kelvin temperature. KEave = 3/2RT where R = 8.3145
"Great Gases Never Pressure Tiny Kettles"
Great = Gases are mostly empty space
Gases = Gas particles are in constant random motion
Never = No attractive or repulsive forces
Pressure = Pressure is due to collisions with the walls
Tiny = Temperature is proportional to average kinetic energy
Kettles = Kinetic energy formula: KEave=32RTKE_{\text{ave}} = \frac{3}{2} RTKEave=23RT
What does being on opposite sides of the PV=nRT equation signify?
Direct relationship
What is kinetic energy? What is the physics equation?
The energy due to motion:
KE=1/2 mv²
Why do we use KEave when discussion gas particles?
A molecules KE vary, and possess different velocities at specific temperatures. As temperature increases, the average velocity of gas molecules increase, which causes KEave to increase
How can you compare the KE and average velocity between two gases?
In general, at constant temperature, the lighter the gas molecules, the faster the average velocity. Temperature can determine KEave if its the same between two gases. Hotter objects have higher KE.
How can you use Graham’s equation to determine the average velocity of gases? How do you determine what M1 is?
V1/V2 = square root of M2/M1
If M1 answer doesnt make sense, put 1/x
What two methods are used to measure relative velocities?
Diffusion: The rate at which gases mix
Effusion: The rate at which gases pass through a tiny hole
both quantities are directly proportional to the average velocity of the gas
How do REAL gases behave?
They do experience intermolecular attractions, causing the pressure that we measure to be less than the ideal pressure
Gas particles have a real volume that may not be negligible. This causes the real velocity of a container to not equate to the actual velocity of empty space that the gas particles can move about
In Van Der Waals equation, explain what a and b is, and why we add/subtract something to pressure and volume in the equation?
(P + an2 /V2 )(V-nb) = nRT
A is related to the strength of intermolecular forces
B is related to the molecular size
We add something to pressure since we usually assume that there’s no intermolecular forces, and we must correct for them
We subtract something from volume to take into account that the actual space that molecules take up.
When does a real gas behave most ideal? why?
At high temperatures and low pressures
High temperatures since gas particles move faster, minimizing the effect of IMF’s between gas particles
Low pressures minimizes the effect of volume on gas particles (lots of empty space)
How can you differentiate between strength of London dispersion forces
Size and shape are two factors that affect the strength of LD. In general, larger covalent compounds have stronger LD forces. The more elongated the molecule, the stronger the LDF
When determining the concentration of ions/what contributes to a solution, what is the one thing you don’t take into account?
The precipitates (SOLIDS)
What is the L/Mol at STP (n)
22.4
What is STP
Standard temperature pressure:
1atm
273.15K
Based off of Boyle’s and Charles’s Law, describe the graphical treatment of each:
What is rate of effusion directly related to?
Velocity
are polyatomics metals or nonmetals
nonmetals
What equation can be used to solve for density using PV=NrT?
PM=dRT