Chapter 3, 4, 6 and 7
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
76 Terms
What are the general categories of amino acid side chains ("R" groups)?
Hydrophobic (nonpolar), Polar (uncharged), Acidic (negatively charged), Basic (positively charged)
What type of side chain does glycine (Gly) have?
Nonpolar; smallest side chain (a single hydrogen)
What is glycine's 3-letter code?
Gly
What type of side chain does alanine (Ala) have?
Nonpolar (hydrophobic)
What is alanine's 3-letter code?
Ala
What type of side chain does valine (Val) have?
Nonpolar (hydrophobic)
What is valine's 3-letter code?
Val
What type of side chain does leucine (Leu) have?
Nonpolar (hydrophobic)
What is leucine's 3-letter code?
Leu
What type of side chain does isoleucine (Ile) have?
Nonpolar (hydrophobic)
What is isoleucine's 3-letter code?
Ile
What type of side chain does methionine (Met) have, and what is unusual about it?
Nonpolar (hydrophobic); contains a sulfur atom
What is methionine's 3-letter code?
Met
What type of side chain does phenylalanine (Phe) have?
Nonpolar (hydrophobic); aromatic ring
What is phenylalanine's 3-letter code?
Phe
What type of side chain does tryptophan (Trp) have?
Nonpolar (hydrophobic); aromatic ring
What is tryptophan's 3-letter code?
Trp
What type of side chain does proline (Pro) have, and what is unusual about it?
Nonpolar (hydrophobic); cyclic structure that links back to amino group
What is proline's 3-letter code?
Pro
What type of side chain does serine (Ser) have?
Polar (uncharged); contains -OH group
What is serine's 3-letter code?
Ser
What type of side chain does threonine (Thr) have?
Polar (uncharged); contains -OH group
What is threonine's 3-letter code?
Thr
What type of side chain does cysteine (Cys) have, and what is unusual about it?
Polar (uncharged); contains a sulfur atom (-SH group)
What is cysteine's 3-letter code?
Cys
What type of side chain does tyrosine (Tyr) have?
Polar (uncharged); contains an aromatic ring and -OH group
What is tyrosine's 3-letter code?
Tyr
What type of side chain does asparagine (Asn) have?
Polar (uncharged)
What is asparagine's 3-letter code?
Asn
What type of side chain does glutamine (Gln) have?
Polar (uncharged)
What is glutamine's 3-letter code?
Gln
What type of side chain does aspartic acid (Asp) have?
Acidic (negatively charged)
What is aspartic acid's 3-letter code?
Asp
What type of side chain does glutamic acid (Glu) have?
Acidic (negatively charged)
What is glutamic acid's 3-letter code?
Glu
What type of side chain does lysine (Lys) have?
Basic (positively charged)
What is lysine's 3-letter code?
Lys
What type of side chain does arginine (Arg) have?
Basic (positively charged)
What is arginine's 3-letter code?
Arg
What type of side chain does histidine (His) have?
Basic (positively charged); imidazole ring
What is histidine's 3-letter code?
His
What determines a protein’s function?
its structure
What structure(s) did Pauling and Corey predict in 1951?
alpha helix and beta sheet
All of the following would disrupt quaternary structure except:
add 8M urea.
treat with beta-mercaptoethanol
decrease the pH
increase the temperature
treat with ascorbic acid (vitamin C)
treat with ascorbic acid (vitamin C)
Your study group is trying to identify differences in the four levels of protein structure. Which of the following would you say is true of important stabilizing forces in secondary structure but not tertiary structure?
The structure is stabilized by ionic attractions between oppositely charge side chains.
The structure is stabilized by H-bonding between polar side chains
The structure is stabilized by hydrophobic interactions between nonpolar side chains
The structure is stabilized by H-bonding between the oxygen of the backbone carbonyl and the hydrogen of the backbone amine.
None of these differentiate between secondary and tertiary structure.
The structure is stabilized by H-bonding between the oxygen of the backbone carbonyl and the hydrogen of the backbone amine.
Why is the peptide bond planar?
It exhibits partial double-bond character, preventing rotation.
Where are beta turns and loops often found?
on the surface of proteins
A clinician friend comes to you and tells you she has a patient that she thinks has some sort of defect in the collagen structure. She wants to know what kinds of structural differences there might be. Which of the following is NOT true for defects leading to scruvy or brittle bone disease?
Proline residues are not hydroxylated
Glycine is replaced by two amino acids
Prolyl hydroxylase activity is deficient
Accumulation of defective collagen causes cell death.
All of the above are true
All of the above are true.
Which of the following amino acid residues would be most likely be buried in the interior of a water-soluble, globular protein?
Aspartate
Lysine
Serine
Phenylalanine
Glutamine
Phenylalanine
Key properties of proteins include:
a) a wide range of functional groups
b) an ability to possess either rigid or flexible structures as dictated by functional requirements.
c) the ability to interact with other proteins
d) A and B.
e) All of the above.
All of the above.
Which of the following secondary structures would you expect to find on the surface of a globular protein?
A) alpha helix
B) beta sheet
C) loops between two alpha-helices
D) none of the above because water would disrupt the hydrogen bonding that stabilizes these structures.
E) A, B, and C as long as the polar and charged amino acid chains face the surface of the protein.
E) A, B and C.
The molecular structure that is short-lived and neither substrate nor product is known as:
transition state
An enzyme will specifically bind its substrate because of:
A tight lock and key binding mechanism
A high number of hydrophobic amino acids in the center of the protein
A large number of weak interactions at the active site
Additional nonprotein cofactors
None of the above
a large number of weak interactions at the active site.
Which of the following is true?
Enzymes force reactions to proceed in only one direction
Enzymes alter the equilibrium of the reaction
Enzymes alter the standard free energy of the reaction.
All of the above.
None of the above.
None of the above.
The active site of an enzyme:
is a series of amino acids that bind the enzyme
is a linear sequence of amino acids that react with each other
bind covalently to the substrate
allows water to enter into the solvate the substrate
None of the above.
None of the above.
At equilibibrium, the gamma G of a rection is:
zero
Riboflavin is a water-soluble organic substance that is not synthesized by humans. Metabolically, it is chemically converted into a substance called flavin adenine dinucleotide, which is required by succinate dehydrogenase. Which of the following statements is MOST correct?
Flavin adenine dinucleotide is a vitamin.
Riboflavin is a coenzyme.
Succinate dehydrogenase is a coenyzeme
Flavin adenine dinucleotide is a coenzyme
Flavin adenine dinucleotide is a coenzyme
A graph of product versus time (as in Fig. 6.2 in your textbook) for an enzyme is determined to be hyperbolic. Why does the amount of product level off as time increases?
The reaction has reached equilibrium, that is, the forward and reverse reactions are occurring at a fixed rate.
What is the common strategy by which catalysis occurs?
stabilization the transition state
The Gibbs free energy of activation is:
the difference between the substrate and the transition state free energies.
Examples of cofactors include:
Zn2+, Mg2+, and Ni2+.
biotin and thiamine pyrophosphate
pyridoxal phosphate and coenzyme A
B and C
All of the above
All of the above.
Allosteric effectors alter the equilibria between:
the R and T forms of a protein.
Homotropic effects of allosteric enzymes:
are due to the effects of substrates.
The KM is:
equal to the product concentration at initial reaction conditions.
equal to the substrate concentration when the reaction rate is half its maximal value
proportional to the standard free energy
all of the above
none of the above
equal to the substrate concentration when the reaction rate is half its maximal value.
Which of the following is true under the following conditions:
the enzyme concentration is 5 nM,
the substrate concentration is 5 mM,
andthe KM is 5 µM.
Options:
The enzyme is saturated with substrate.
Most of the enzyme does not have substrate bound.
There is more enzyme than substrate.
All of the above.
None of the above.
The enzyme is saturated with substrate.
When substrate concentration is much greater than KM, the rate of catalysis is almost equal to:
V max
Allosteric enzymes:
contain distinct regulatory sites and have multiple functional sites.
display cooperativity
have distinct regulatory sites but still show Michaelis-Menten kinetics
A and B.
A, B and C.
A and B.
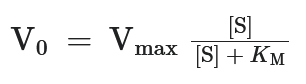
This formula describes:
Michaelis-Menten plot
A critical feature of the Michaelis-Menten model of enzyme catalysis is:
formation of an ES complex.
When reaction conditions are such that the amount of substrate is far greater than the amount of enzyme present, then the following condition is also met (choose one).
The [substrate] is much less than KM
The V0 is half Vmax
The enzyme is displaying second-order kinetics
The enzyme is displaying first-order kinetics
The enzyme is displaying zero-order kinetics
The enzyme is displaying zero-order kinetics
Allosteric effectors:
can lead to a decrease in the availability of a protein
can cause large changes in enzymatic activity
alter enzyme activity by binding to the active site of an enzyme
decrease the sensitivity of the enzyme at nearly all concentrations of substrate
do not alter the sensitivity of a metabolic pathway
can cause large changes in enzymatic activity
What two biochemical principles explain the enzyme activity versus temperature curve?
The rising portion of the curve is due to increase in enzyme synthesis, and the decrease is due to activation of inhibitor molecules
The rising portion of the curve is due to increase in Brownian motion of the molecules, and the decrease is due to enzyme denaturation
The rising portion of the curve is due to increase in enzyme synthesis, and the decrease is due to reduction in Brownian motion of the molecules
An increase in temperature increases the interactions with allosteric activators, and a decrease in temperature increases the interactions with allosteric inhibitors
The rising portion of the curve is due to increase in Brownian motion of the molecules, and the decrease is due to activation of inhibitor molecules
The rising portion of the curve is due to increase in Brownian motion of the molecules, and the decrease is due to enzyme denaturation
What type(s) of inhibition are reversable?
competitive
noncompetitive
uncompetitive
All of the above.
None of the above
All of the above.
In what type of inhibition can the inhibitor only bind to the ES complex?
irreversible
uncompetitive
noncompetitive
competitive
None of the above.
uncompetitive
What conclusion can be drawn concerning an inhibitor if the KM is the same in the presence and absence of the inhibitor?
The Vmax is larger in the presence of inhibitor.
The inhibitor binds to the same site as the substrate.
The inhibitor binds to the substrate.
The inhibitor forms a covalent bond with the enzyme.
The inhibitor has a structure that is not very similar to the substrate.
The inhibitor has a structure that is not very similar to the substrate.
Which of the following is a type of irreversible inhibitor?
uncompetitive inhibitor
allosteric inhibitor
competitive inhibitor
group-specific reagent
non-competitive inhibitor
group-specific reagent