exam 4 + exam 5 mcb 181
1/199
Earn XP
Description and Tags
NOT CREATED BY ME -- created by Victoria_Minutillo2 on quizlet, i just refuse to pay for something that used to be free. i will not be swayed by corporate greed.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
200 Terms
Proteins function:
-structure (keratin, collagen, elastin)
-catalyse (enzymes)
-carries & transport (membrane channels)
-receptors & binding (defense)
-contraction (actin & myosin)
-signaling (hormones)
-storage (bean seed proteins)
-immune response (immunoglobulin/antibodies)
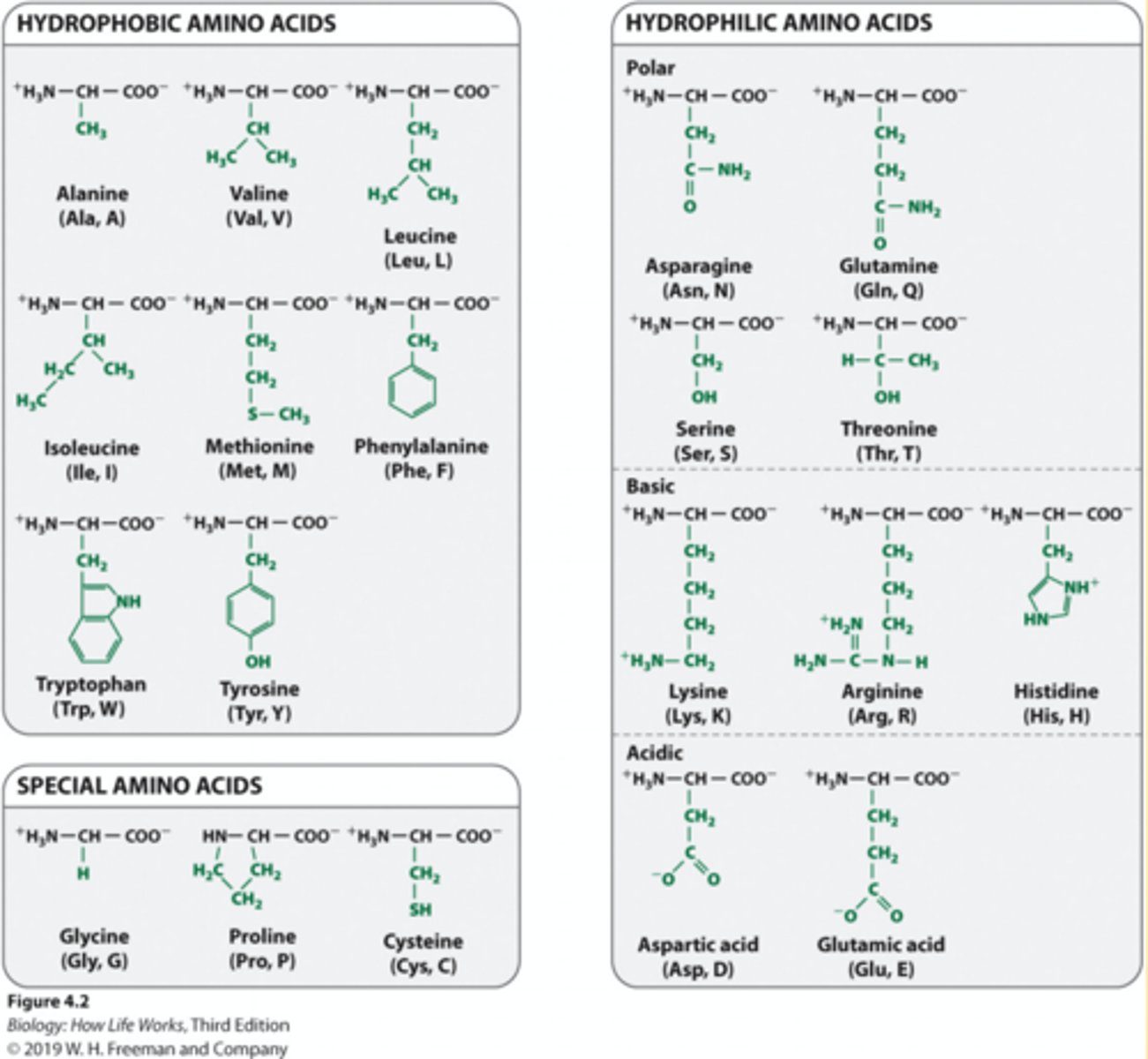
amino acid structure
1. Proteins and polymers are made of monomers called amino acids.
2. Amino acids consist of a central carbon atom (α carbon).
3. The central carbon atom is covalently bonded to amino and carboxyl functional groups, a hydrogen, and a side chain or R group.
4. The R groups make each amino acid unique and are responsible for the chemical and physical properties of each amino acid monomer

Structure of the 20 amino acids
1. The R groups allow the 20 amino acids to be grouped easily based on the following characteristics: a. How they interact with water (hydrophilic or hydrophobic) b. Whether they are basic or acidic c. Whether they are polar or non-polar
2. These properties are very important because they influence how a protein folds and its three-dimensional shape.
3. Shape = function.
peptide bonds
Covalent bonds form between amino acid monomers
peptide bonds formation
a. The carboxyl group of one amino acid reacts with the amino group of another amino acid.
b. A molecule of water is released via a dehydration (condensation) synthesis reaction.
2. The free amino group is at the amino end of the peptide, and the carboxyl group is at the carboxyl end.
-The sequence of amino acids dictates how proteins fold in three dimensions
-Based on sequence of amino acids and the order of the R groups, the proteins will fold in a particular way. another.
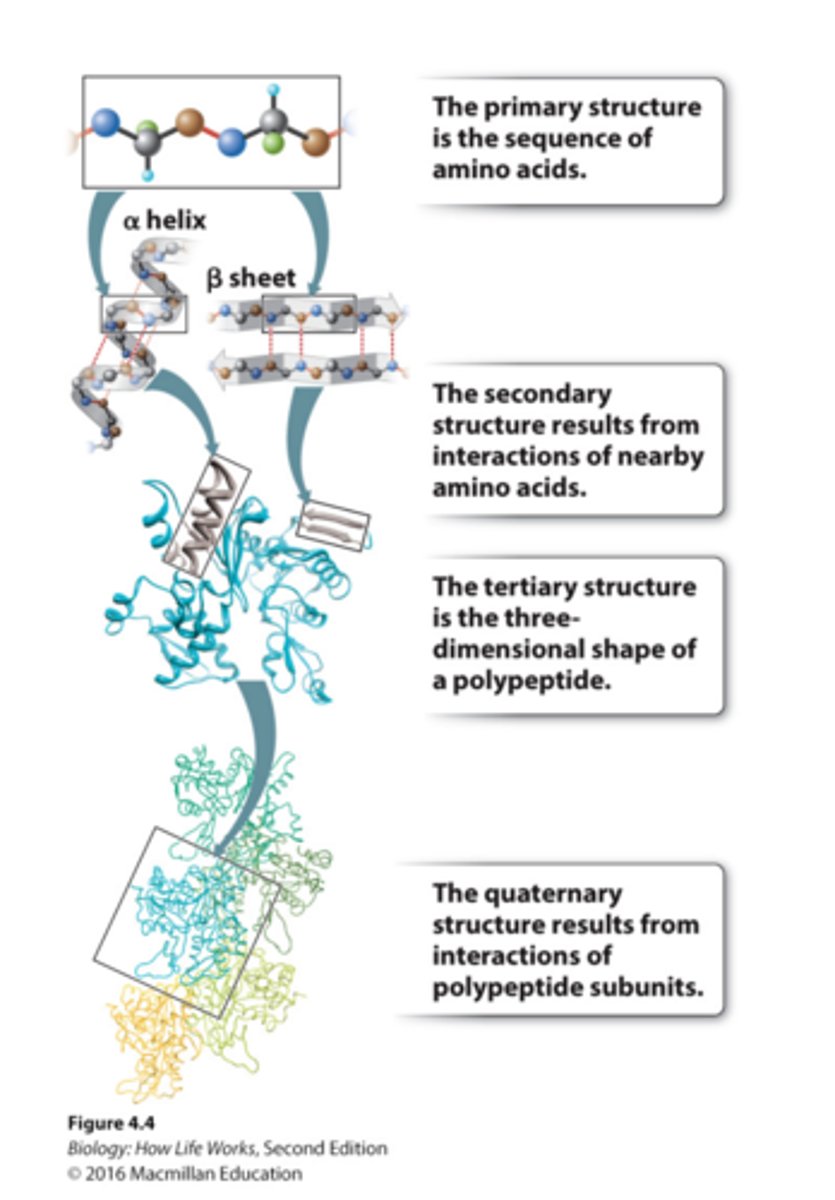
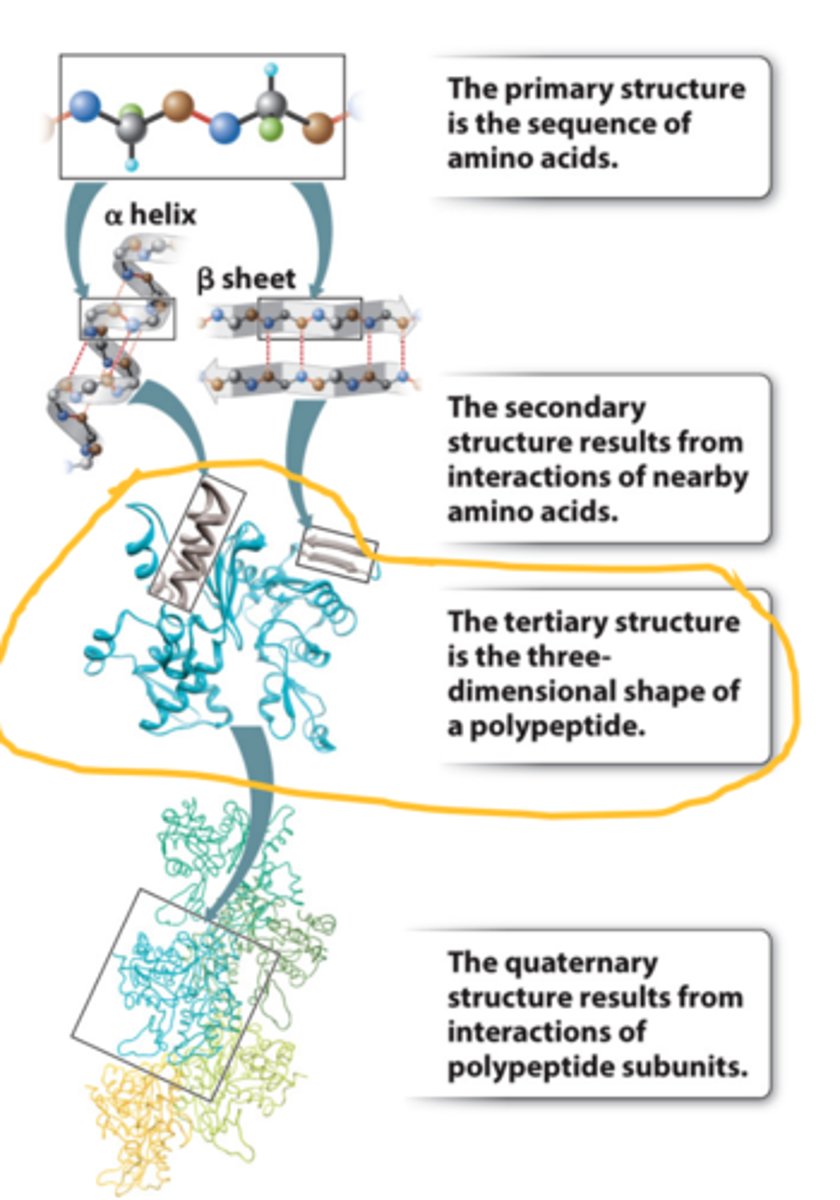
primary structure of protein
1. The primary structure is the amino acid sequence.
2. It can be represented by a series of three-letter or one-word abbreviations.
3. They are listed starting at the amino end (N-terminus) and going toward the carboxyl end (C-terminus).

secondary structure
-Secondary structure results from hydrogen bonding between backbone groups
-Alpha helices are common secondary structures in proteins
-Beta sheets are common secondary structures in proteins
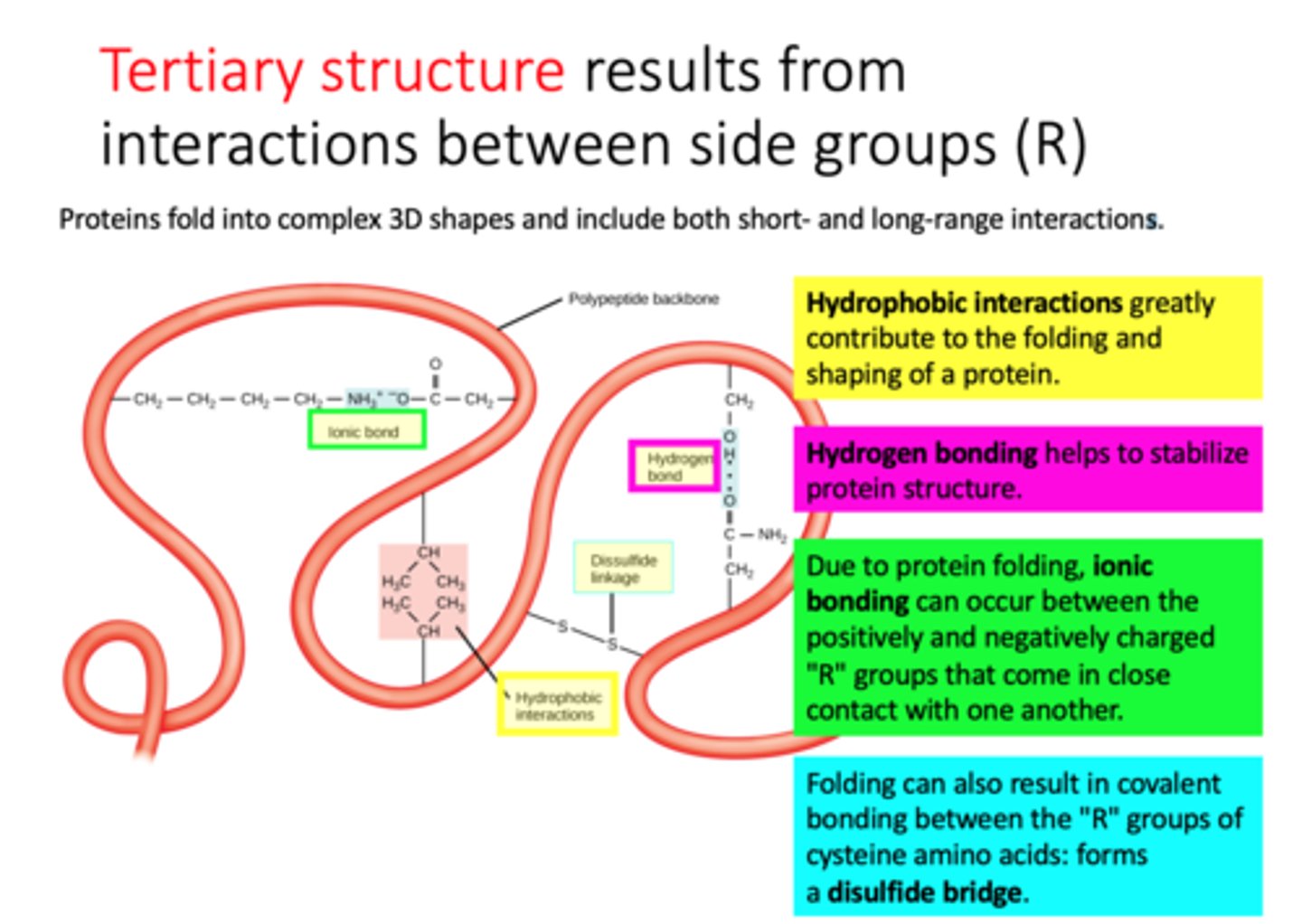
tertiary structure
-results from interactions between side groups (R)
-3D shape of polypeptide, usually made of several secondary structure elements.
-The tertiary structure is determined by the spatial distribution of the hydrophilic and hydrophobic R groups along the molecule as well as by the chemical bonds and interactions that form between the R groups.
three ways to illustrate tertiary structure
a. Ball-and-stick model: shows the atoms in the amino acid chain
b. Ribbon model: emphasizes the alpha helices and the beta sheets
c. Space-filling model: shows the shape and contour of the folded protein
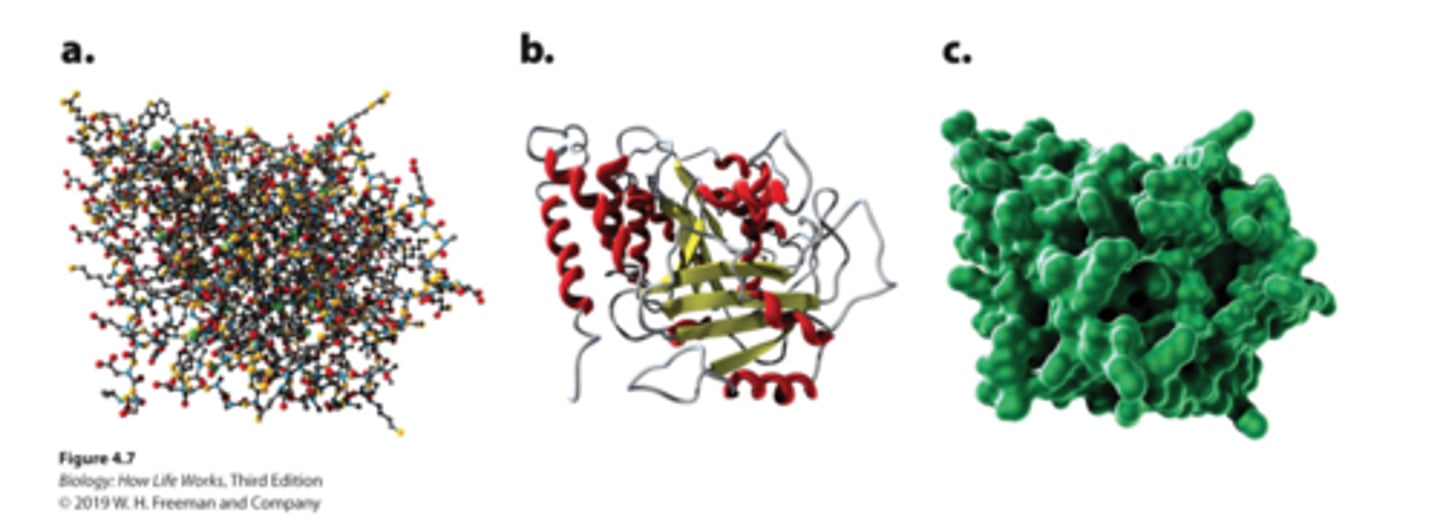
diagram of structure of protein with amino acid sequence
What influences proteins' final folding?
-Most protein folding is driven primarily by hydrophobic interactions: hydrophobic side chains cluster together in the center of the protein, avoiding the surrounding water. Backbone and side chain interactions help to maintain the basic structure.
-Most proteins can fold into their proper shapes spontaneously in water at pH 7, although in cells, there are often proteins (chaperones) that help the process along
Most protein folding is driven primarily by hydrophobic interactions: hydrophobic side chains cluster together in the center of the protein, avoiding the surrounding water. Backbone and side chain interactions help to maintain the basic structure?
Valine
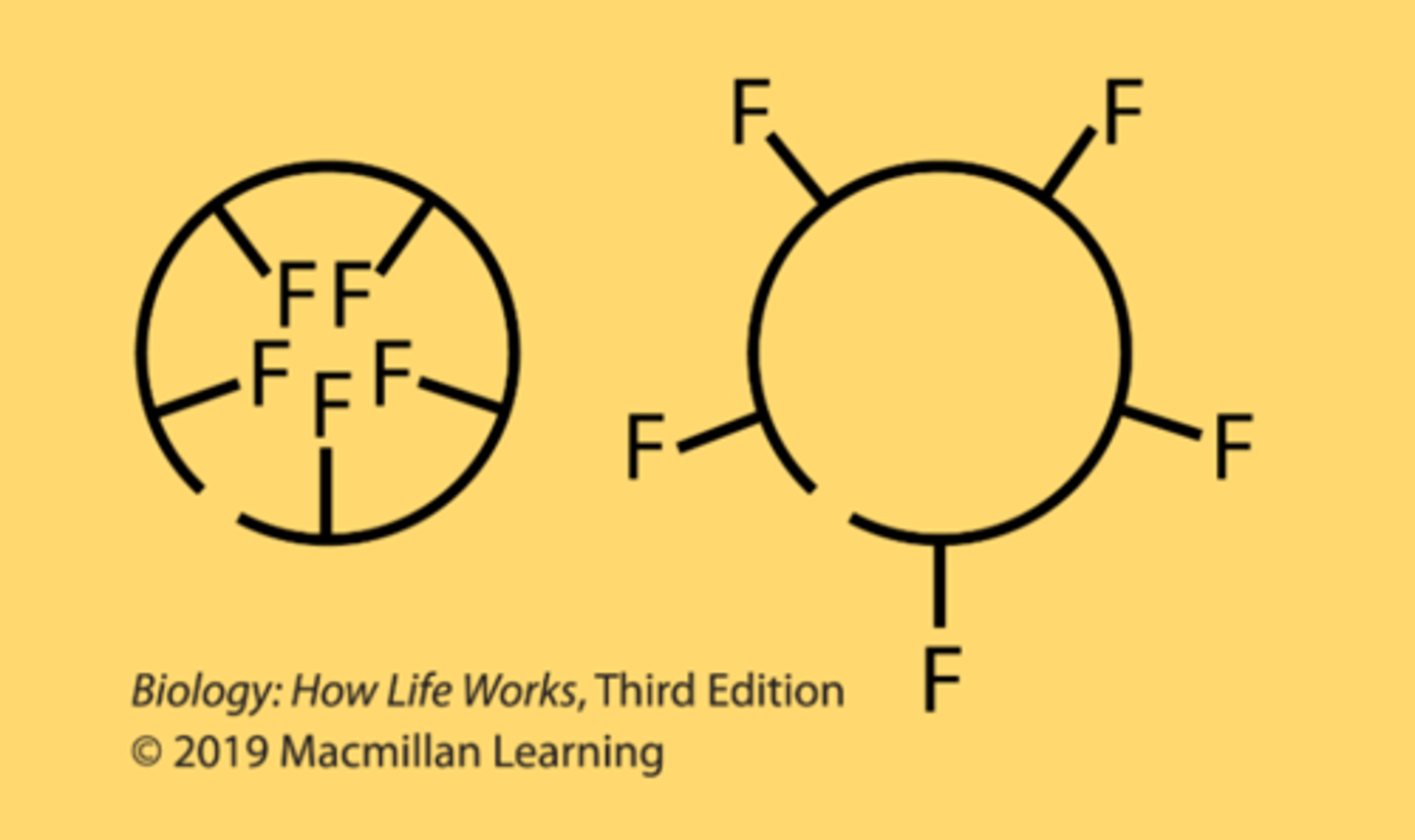
A folding domain of a polypeptide chain has a primary structure containing 5 phenylalanine residues, where F represents the side chain of phenylalanine. Consider the possible folding orientations. If this domain folds in one of the two orientations shown, which is more likely, the one on the left or the one on the right?
left one
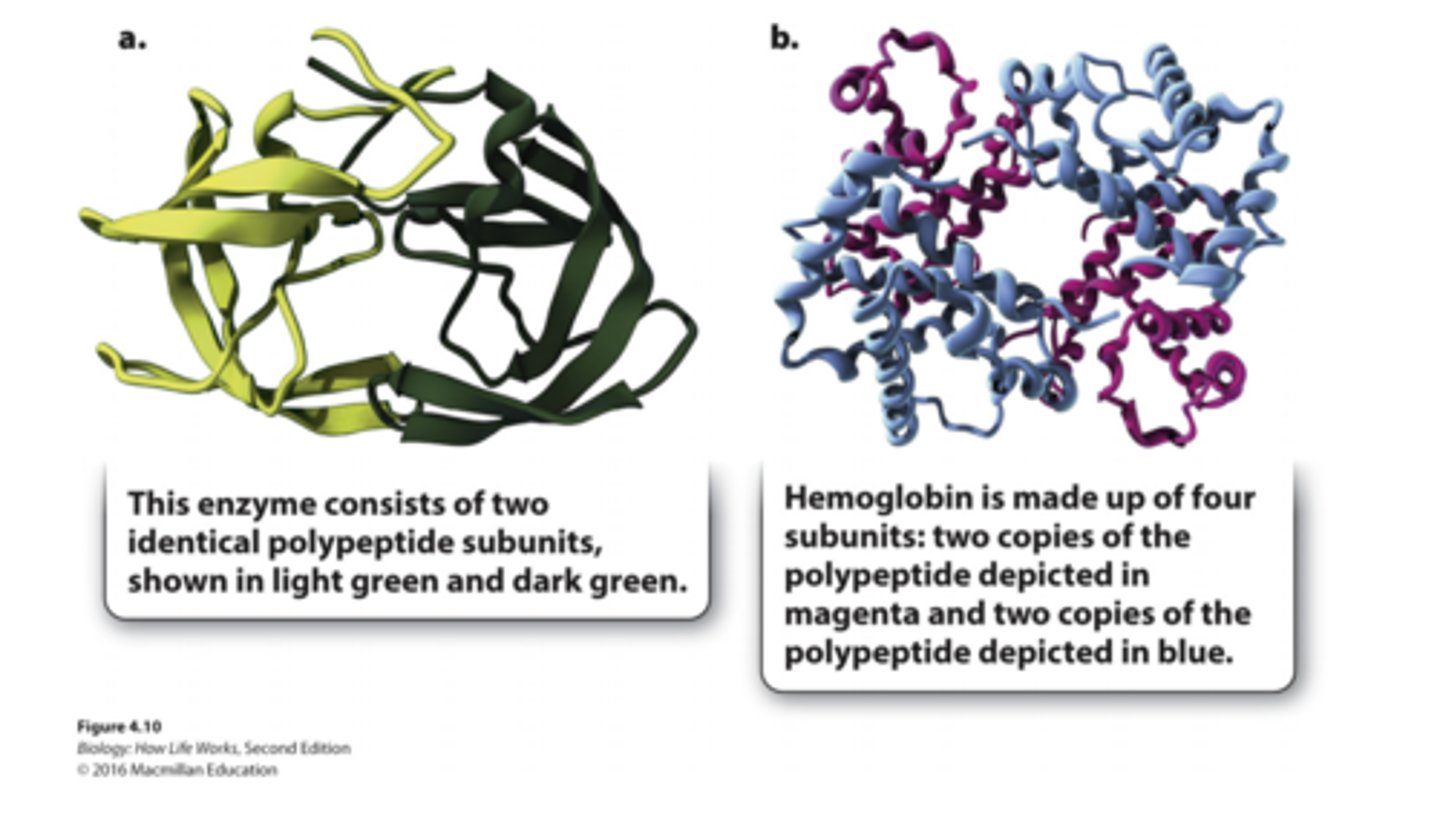
quaterny structure
-structure results from interactions of polypeptide subunits
Which force can contribute to a protein's secondary structure?
hydrogen bonding
Why proteins final folding is important?
1. Ultimately, the primary structure determines the secondary and tertiary structures of a protein
2. The tertiary structure determines the protein's function: the contours and distribution of charge on the outside of the molecule and the presence of pockets that might bind with smaller molecules on the inside all play a role in the protein's function.
bacterial protein (shown as a spacefilling model)
has a cavity that can bind with a small molecule (shown as a balland-stick model in the center).
Hemoglobin
made up of four subunits:
two copies of polypeptide depicted in magenta & two copies of polypeptide depicted blue
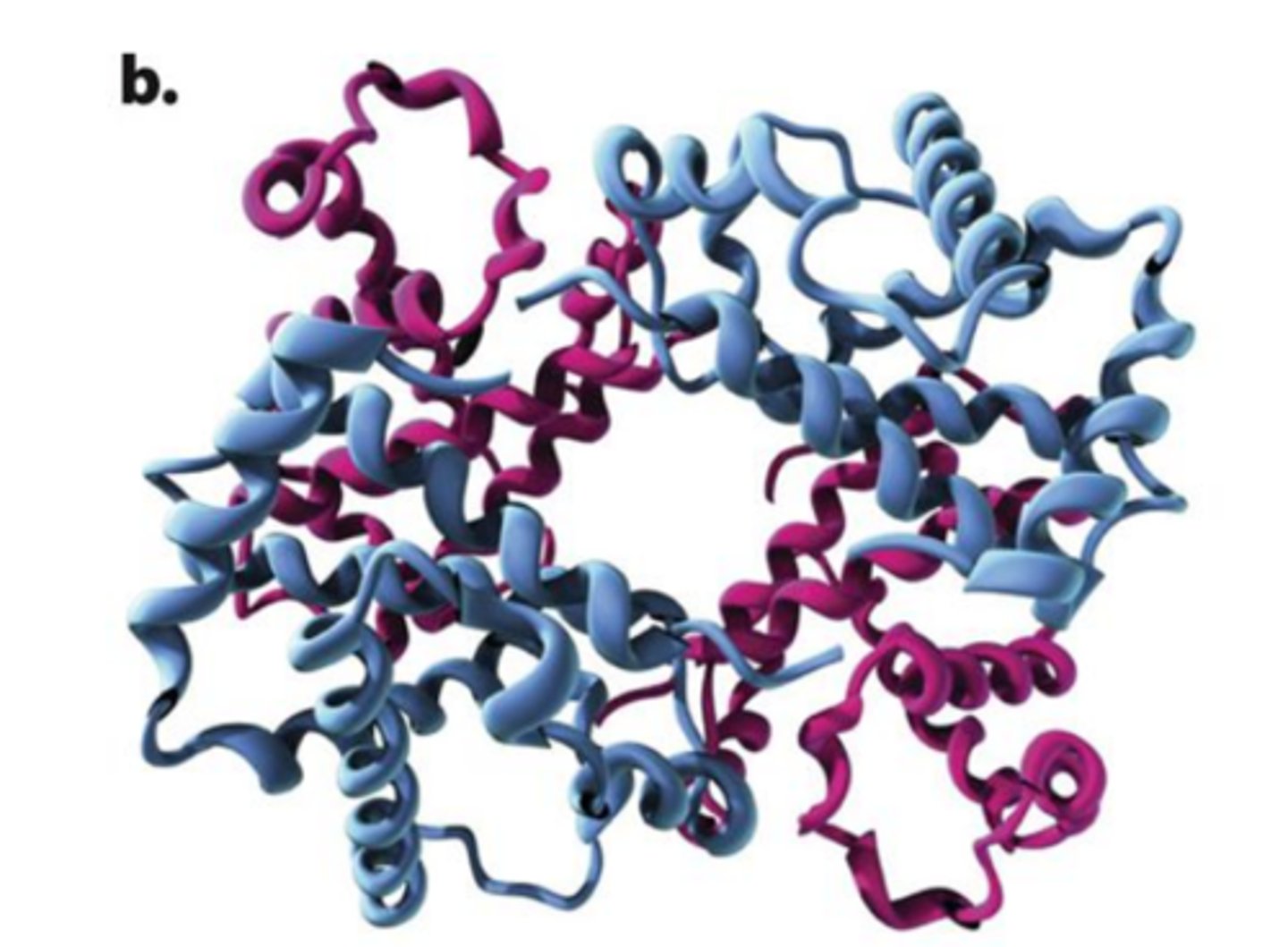
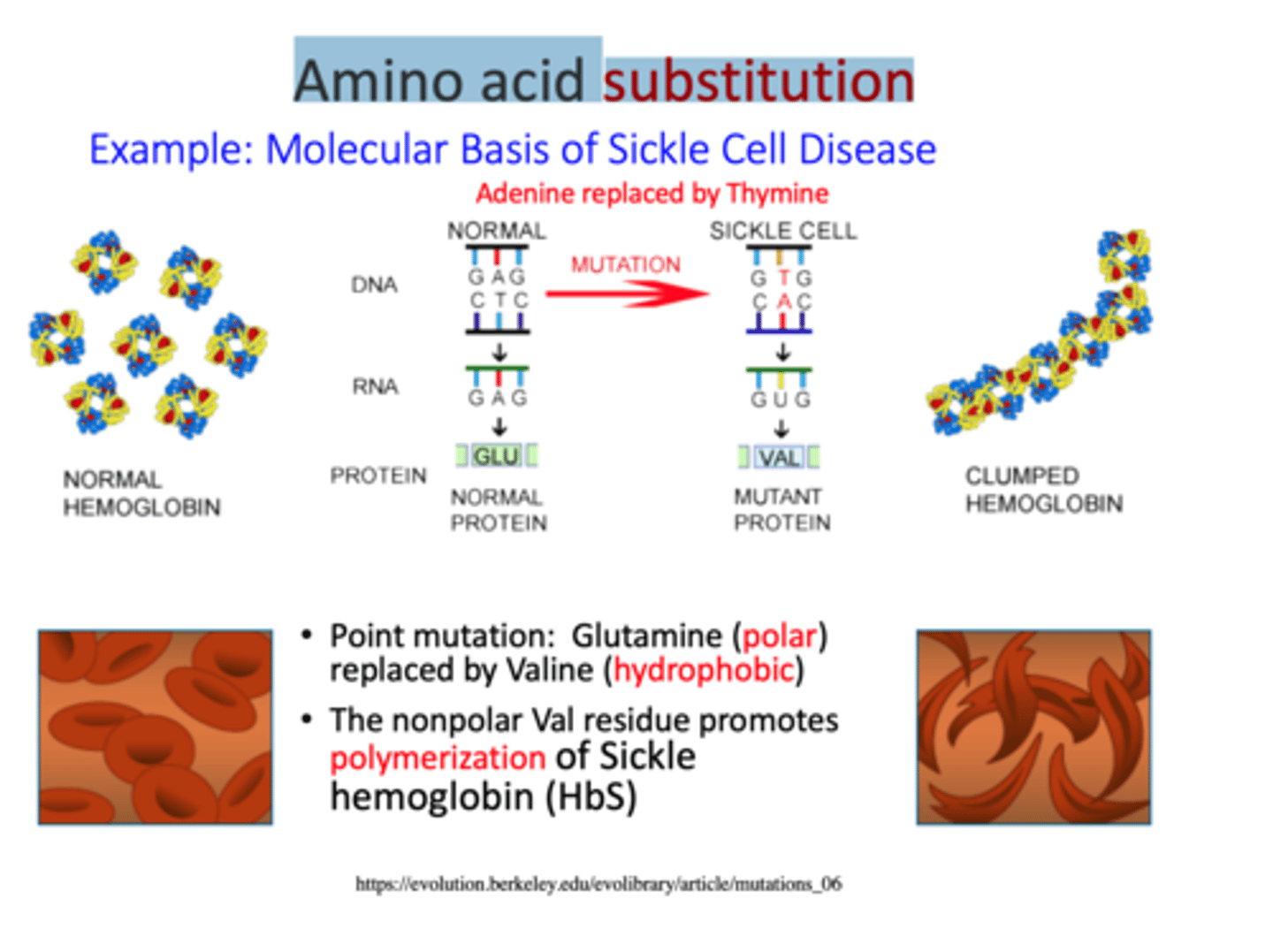
Sickle-cell anemia is due to a single amino-acid replacement
-single glutamate to be replaced by a valine in one of the polypeptides that makes up the hemoglobin protein. Half of the hemoglobin molecules in his body have this substitution.
-valine sits where gluatmate acid should be and makes hemoglobin molceules stick together forming long fiber disort shape of red blood cells
Amino acid substitution
A mutation that results in replacement of the correct amino acid with an incorrect amino acid
Same genotype can result in multiple phenotype. Same phenotype can be encoded by multiple genotypes
true
A protein you work on has a very important glutamic acid (glutamate) residue in the site where it binds to its substrate. This helps it to bind the substrate and catalyze a chemical reaction.
Which of the following amino-acid substitutions would likely have the least impact on the function of this protein?
Replacing that glutamate with an aspartate
What happens if protein structure comes apart?
1. If a protein loses its structures, it also loses its function.
2. Proteins can be denatured (unfolded) by chemical treatment (e.g. change in pH, high salt) or high temperatures and lose their function.
3. If the optimal conditions are returned, the protein can refold and regain its function
Protein denaturation
Under changes in temperature or pH, or exposition to chemicals or UV, H bonds and interactions between R groups may be disrupted, causing the protein to lose its three-dimensional structure and turn back into an unstructured string of amino acids: Denatured proteins are usually non-functional. (can be reversed)
How is denaturing proteins similar and/or different from denaturing nucleic acids?
Heat can denature both proteins and nucleic acid secondary structure (e.g. double helix) because each held together by non-covalent interactions
reuqirements of the cell
-A way to encode/transmit informationA membrane separating the inside of the cell from the outside
-ENERGY
Adenosine Triphosphate (ATP)
1.ATP stores energy in a form that all cells can readily use to perform the work of the cell.
2.Hydrolysis of ATP drives many reactions in cells.

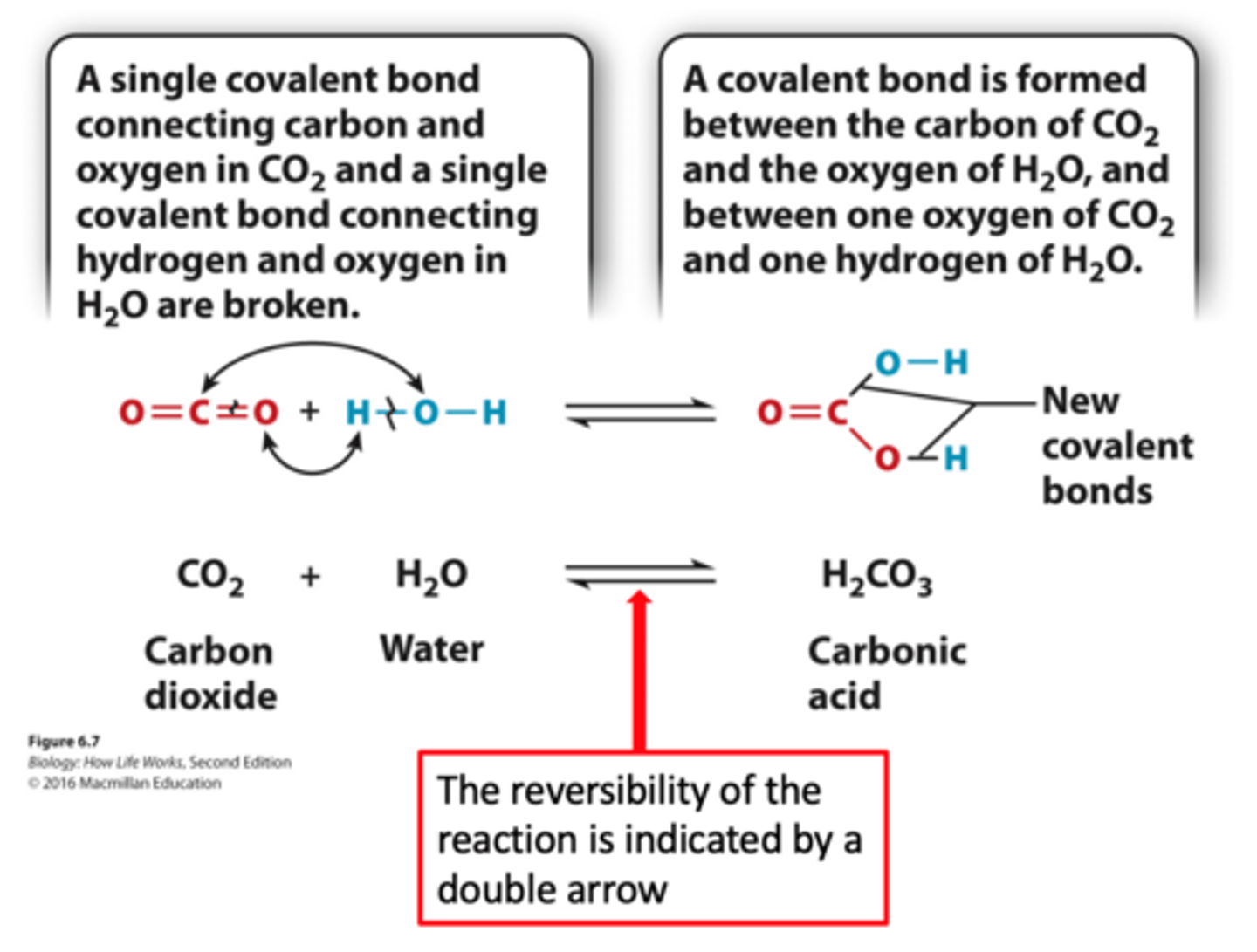
chemical reactions
-When chemical reactions occur, bonds between atoms are broken and new bonds are formed. The reversibility of the reaction is indicated by a double arrow
-Many chemical reactions in cells are readily reversible.
Gibbs Free Energy (ΔG)
= amount of energy in a system available to do work
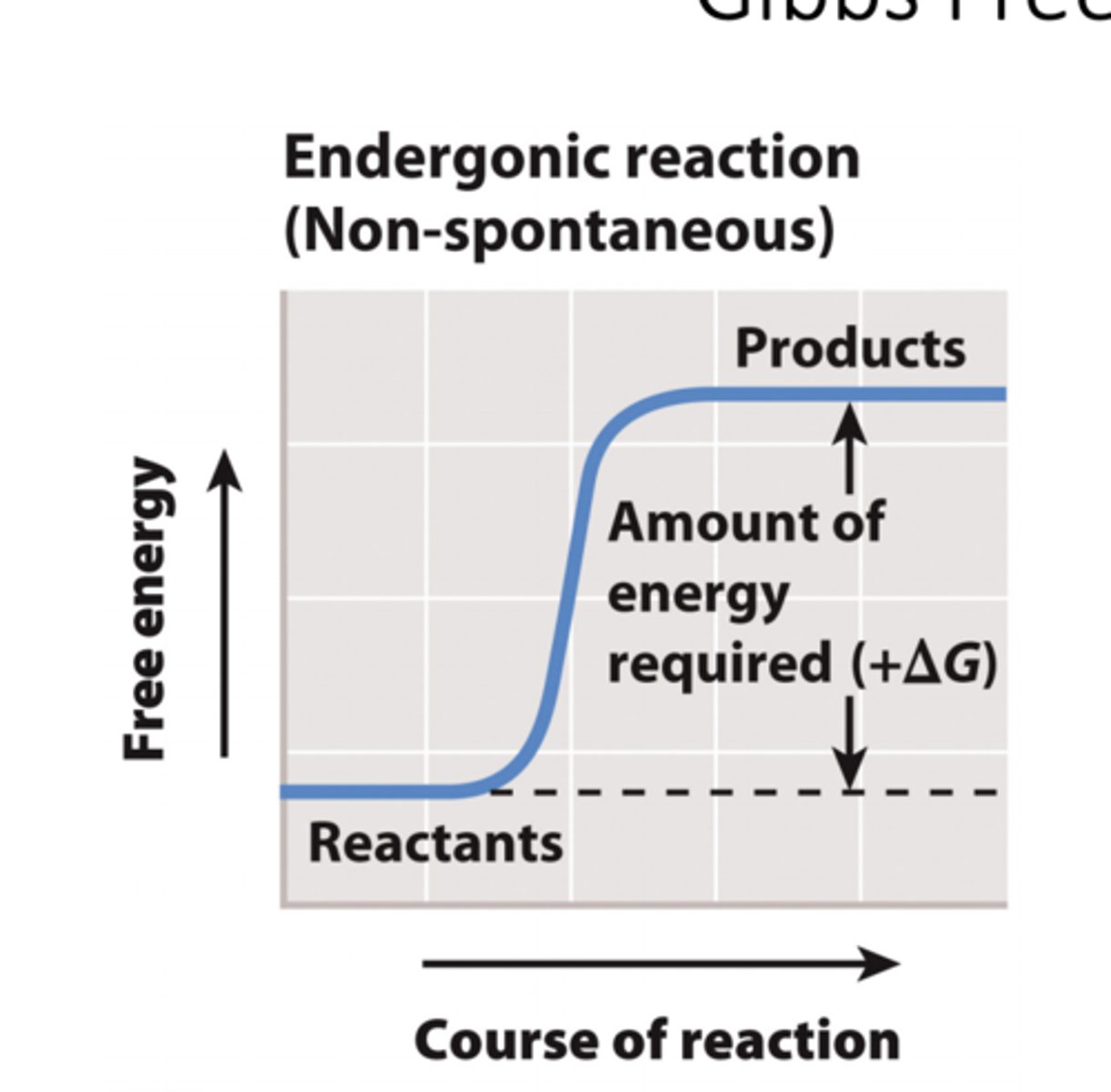
endergonic reactions
1.The difference in Gibbs free energy between the reactants and the products of a chemical reaction is written as ΔG.
2. If the products of a reaction have more free energy than the reactants, then ΔG is positive.
3. If the reactants have more free energy than the products, then ΔG is negative.
4. Reactions with a positive ΔG require an input of energy and are endergonic.
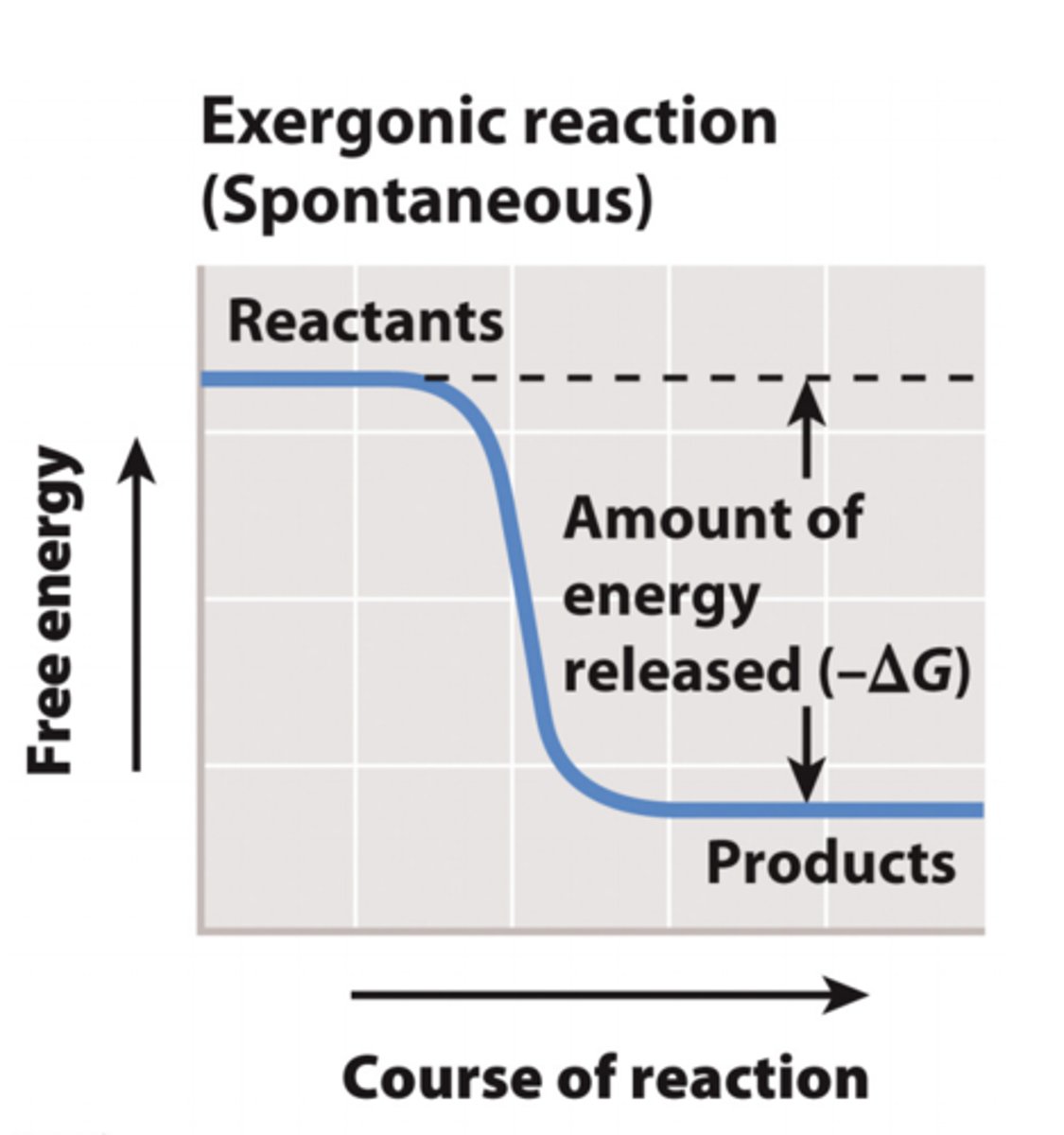
exergonic reactions
1. Reactions with a negative ΔG release energy and are exergonic.
2. These reactions occur spontaneously.
3. Spontaneous does NOT mean that a reaction will occur quickly- it means it will occur without a net input of energy.
example of exergonic reactions
ATP hydrolysis is an exergonic reaction
∆G = -7.3 kcal/mole
Which of the reactions is most likely to be exergonic?
-the digestion of protein from food into amino acids
-larger complex molecule -> smaller molecules
coupled reactions
Non-spontaneous reactions are often coupled to spontaneous reactions: Coupled Reactions
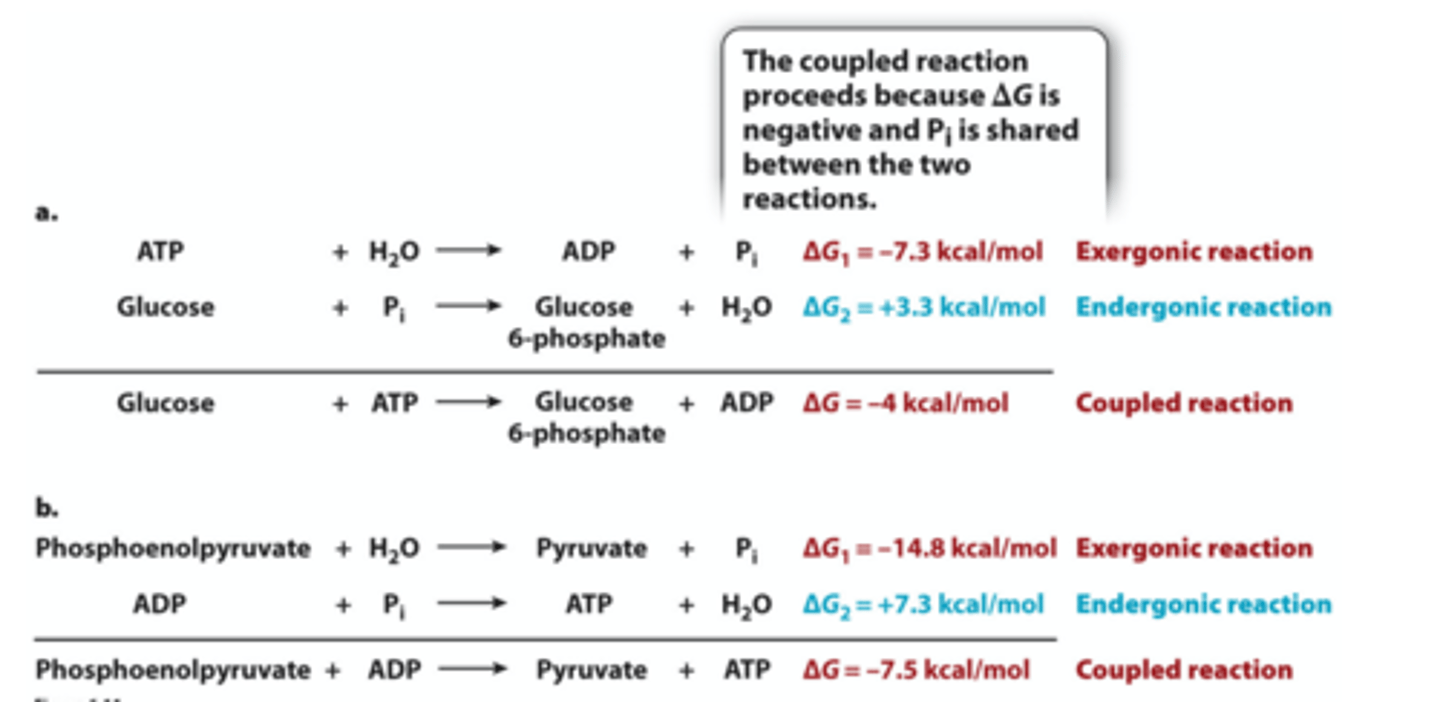
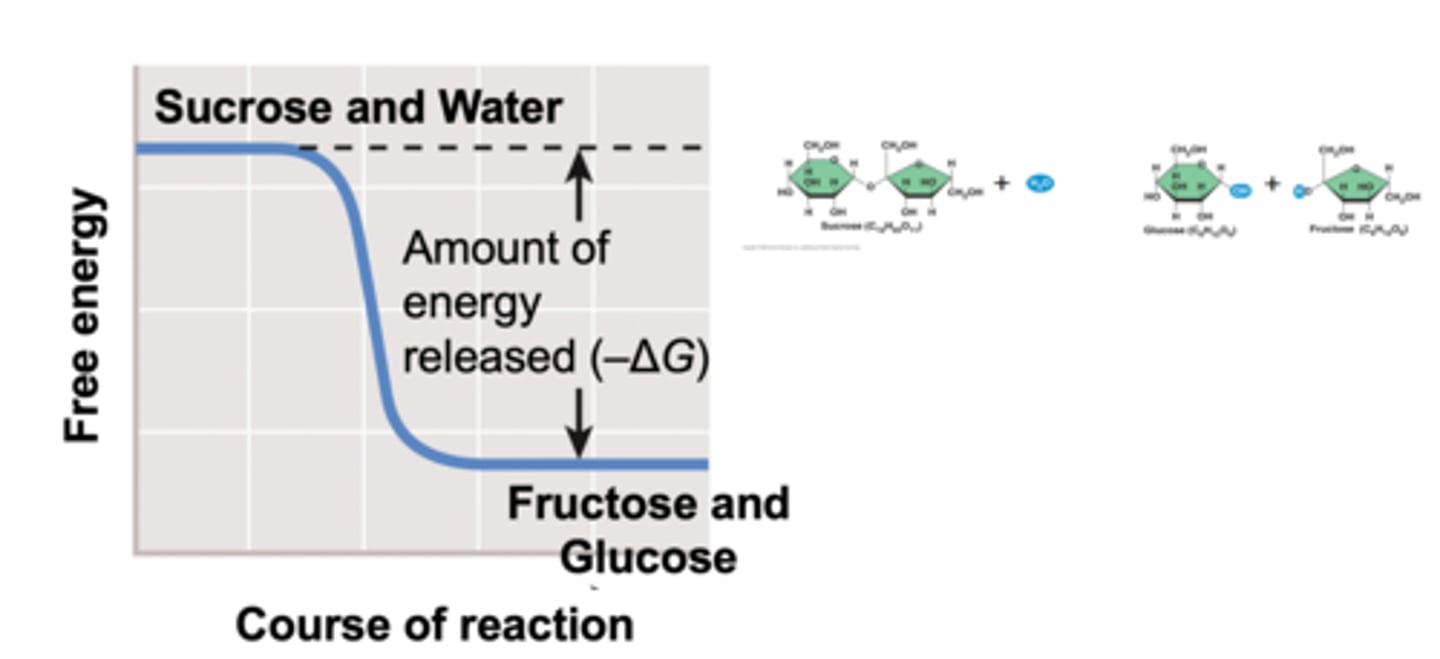
example of coupled reactions: Digestion of Sucrose
-Hydrolysis of the glycosidic bond releases two monosaccharides: glucose and fructose
-The total overall reaction is EXERGONIC
-Outside living organisms, sucrose breakdown occurs VERY SLOWLY
hydrolysis of sucrose
exergonic reactions
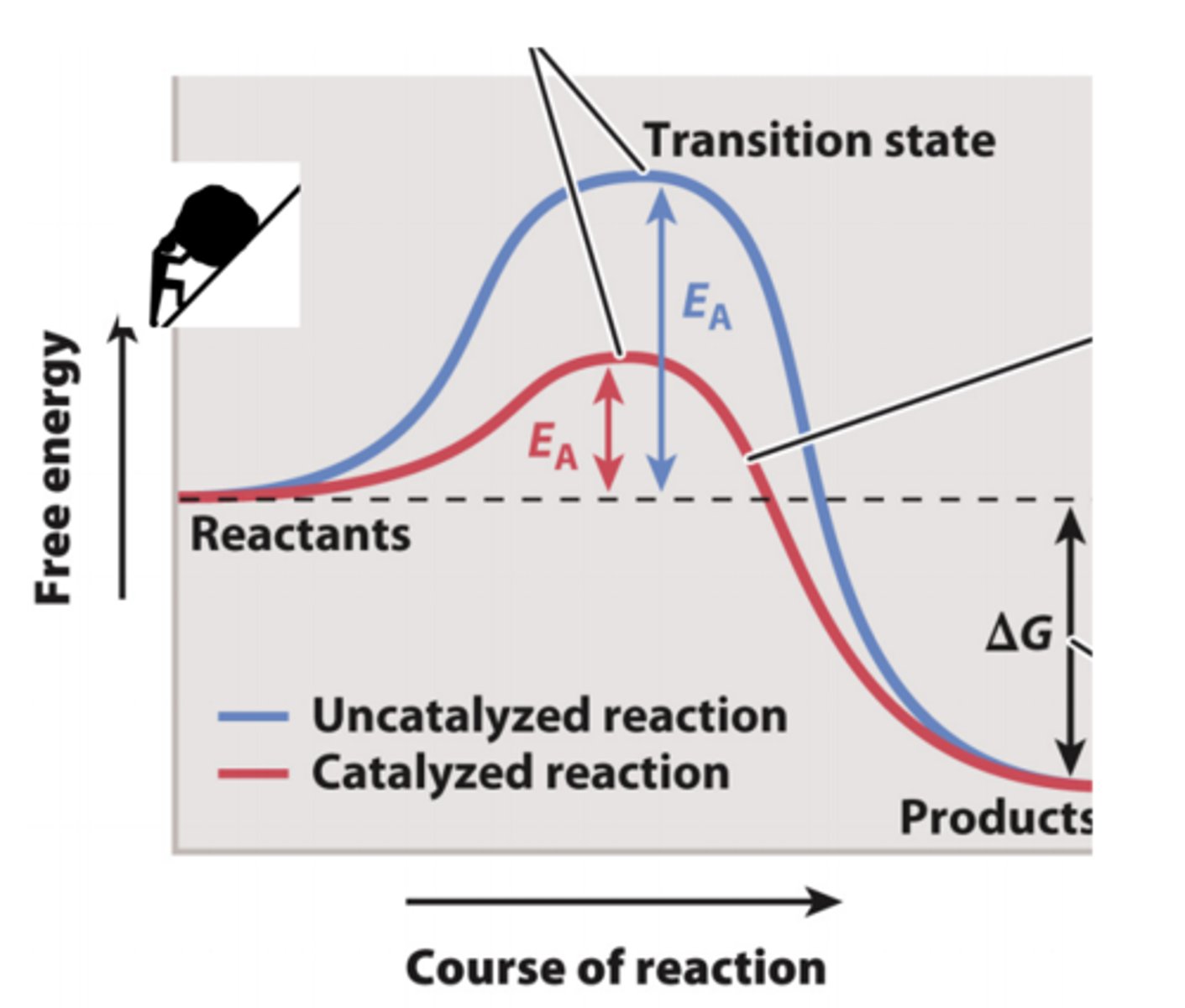
Free-Energy Change & Activation Energy
-Even exergonic reactions don’t always proceed very quickly on their own because they have to get over an energy barrier in order to proceed.
-This is because in order to get from reactants to products, the reacting atoms have to go through a transition state that actually has higher free energy than the reactants. This is called the activation energy.
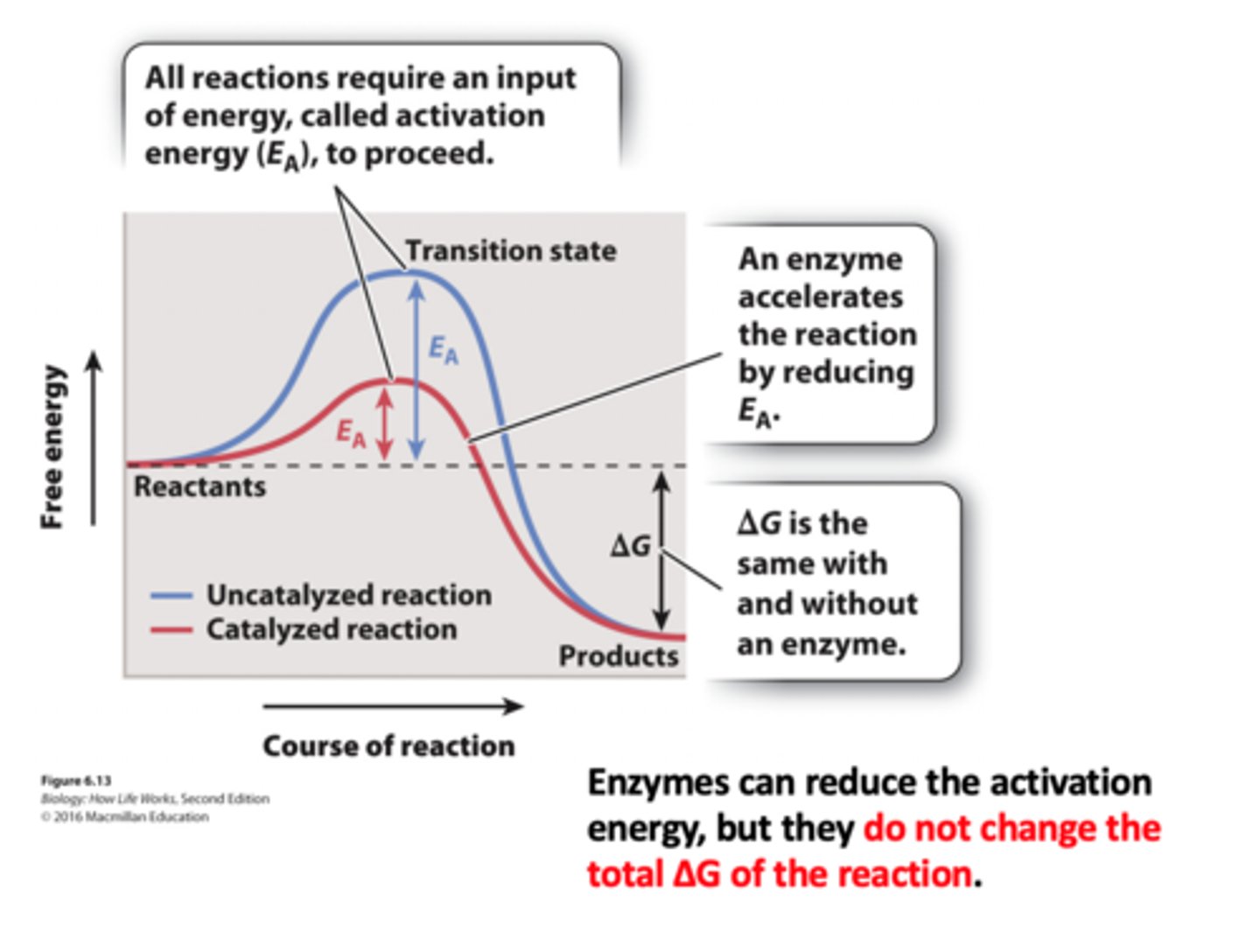
Enzymes catalyze reactions by reducing the activation energy
1. When the activation energy is low, the reaction is faster.
2. Enzymes are able to reduce the activation energy by stabilizing the transition state.
3. The rate of the reaction increases because the activation energy is reduced.
Enzymes also catalyze coupled reactions
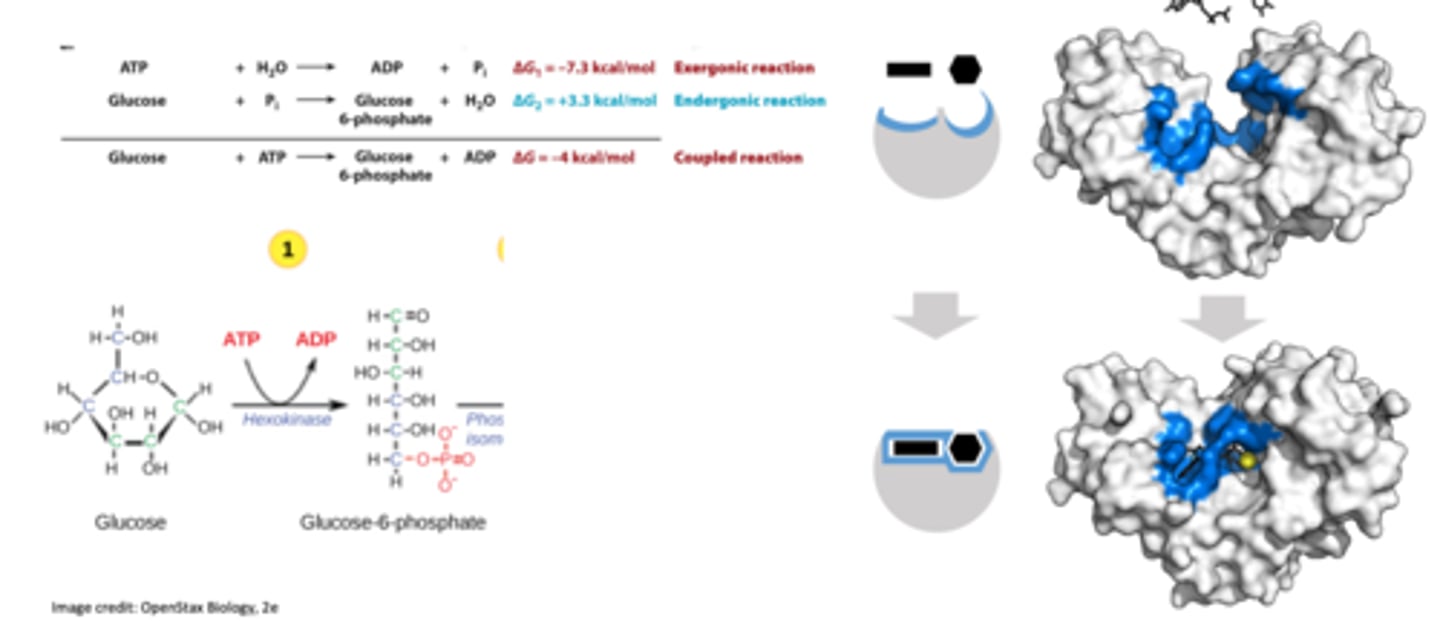
Which energy change labeled by a lower case letter (a-e) is the ∆G of the reaction?
D
when chemical reactions occur the _______ but the _______.
atoms retain their identities; arrangement of bonds changes
when chemical reactions occur the ______ but the ________
pairing of atoms that share electron pair changes; individual atomic nuclei stay the same
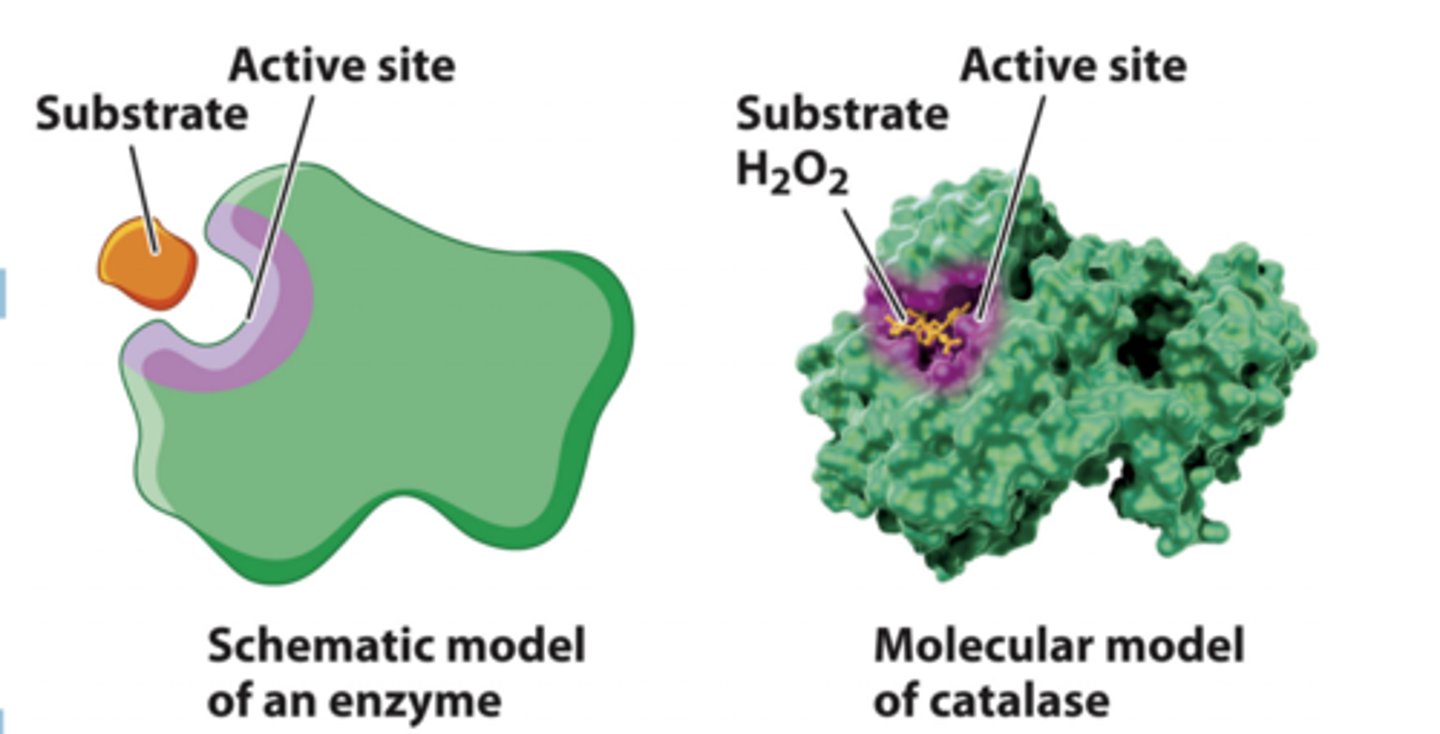
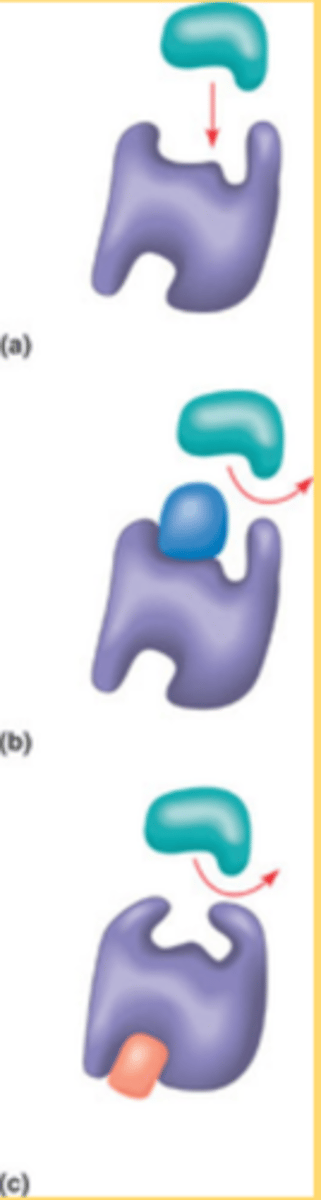
enzymes bind substrates at the active site
1. Enzymes have a 3D structure that brings together particular amino acids to form the active site.
2. The enzyme active site binds the substrate and converts it to the product.
3. The interactions between the substrate and the active site are weak noncovalent interactions or transient covalent bonds that stabilize the transition state and decrease the activation energy required for the reaction
characteristics of enzymes
-Allow chemical reactions to occur at the rate needed for cells to survive and thrive
-Specific
-Unchanged by the chemical reaction
-Generally proteins (but can be nucleic acid)
-Have optimal pH, temperature, and ionic strength
-Can be regulated C
active site of enzyme
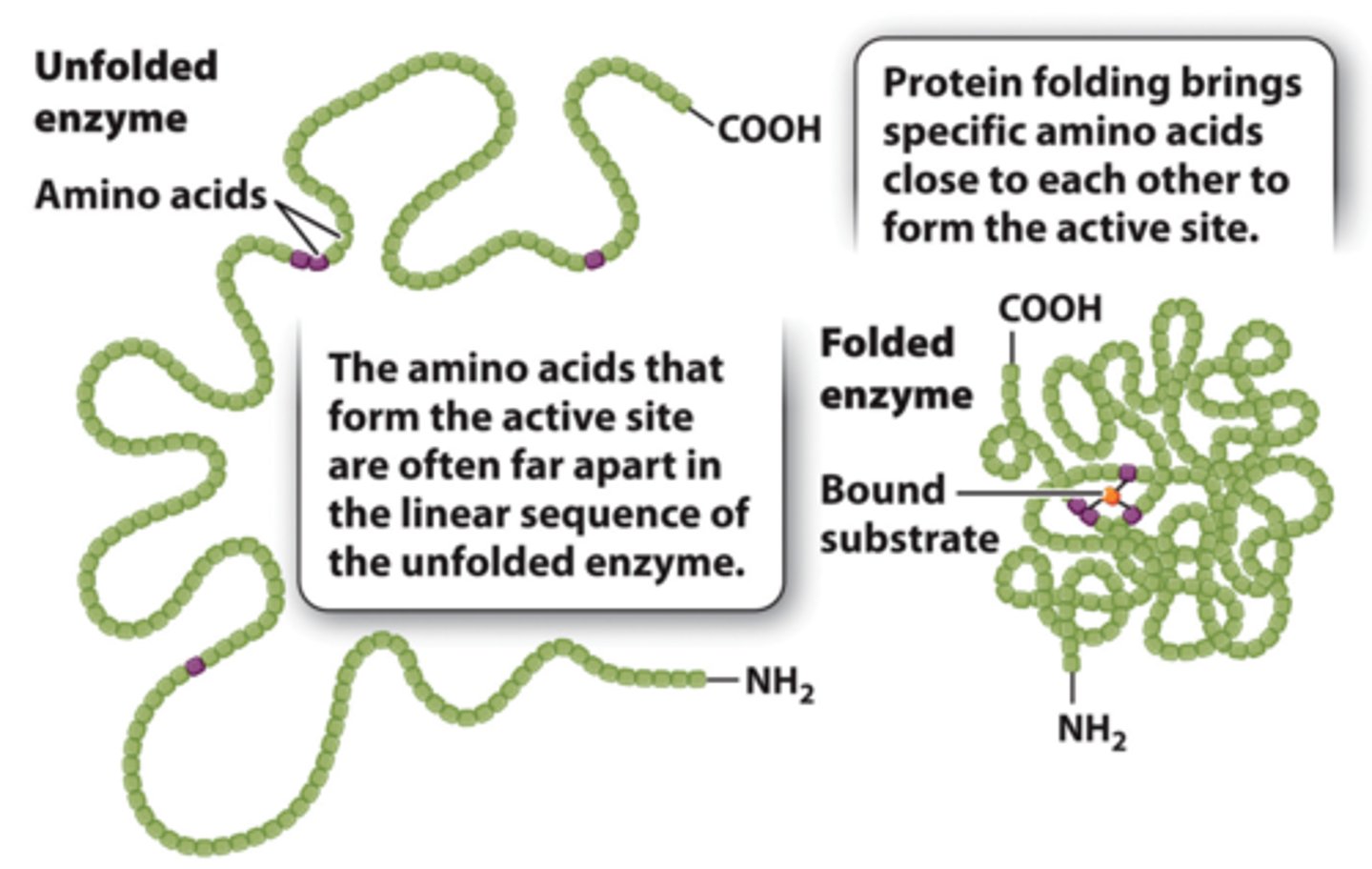
1. An enzyme's active site is extremely small compared to the enzyme itself.
2. The active-site amino acids may be spaced far apart in the primary sequence of the enzyme, but when the protein is folded, they come together to form the active site.
3. Enzymes active site is specific for both the substrate and the type of reaction that is catalyzed.
enzymes catalyze ______ & ________
reactions & coupled reactions
Enzymes increase reaction rates of the reactions they catalyze in two primary ways:
-Enzymes physically align reactants in the orientations needed for reactions to proceed.
-Enzymes stabilize the transition states of reactants through temporary chemical interactions.
Which of the following reactions would you predict could be coupled to ATP synthesis from ADP + Pi (ADP + Pi → ATP + H2O, ΔG + 7.3 kcal/mol)?
creatine phosphate + H2O → creatine + Pi , ΔG - 10.3 kcal/mol
Experimental Demonstration of the Enzyme/Substrate Complex
1. The enzyme β-galactosidase catalyzes the cleavage of the glycosidic bond that links galactose to glucose in the disaccharide lactose.
2. Lactose belongs to a family of molecules called β-galactosides: it contains a galactose unit attached to the rest of the molecule by a glycosidic bond.
3. In a related compound called βthiogalactoside, the oxygen atom in the glycosidic bond is replaced by sulfur.
4. The enzyme β-galactosidase binds βthiogalactoside but cannot cleave or release it.
Demonstration of the Enzyme/Substrate Complex: Experiment 1
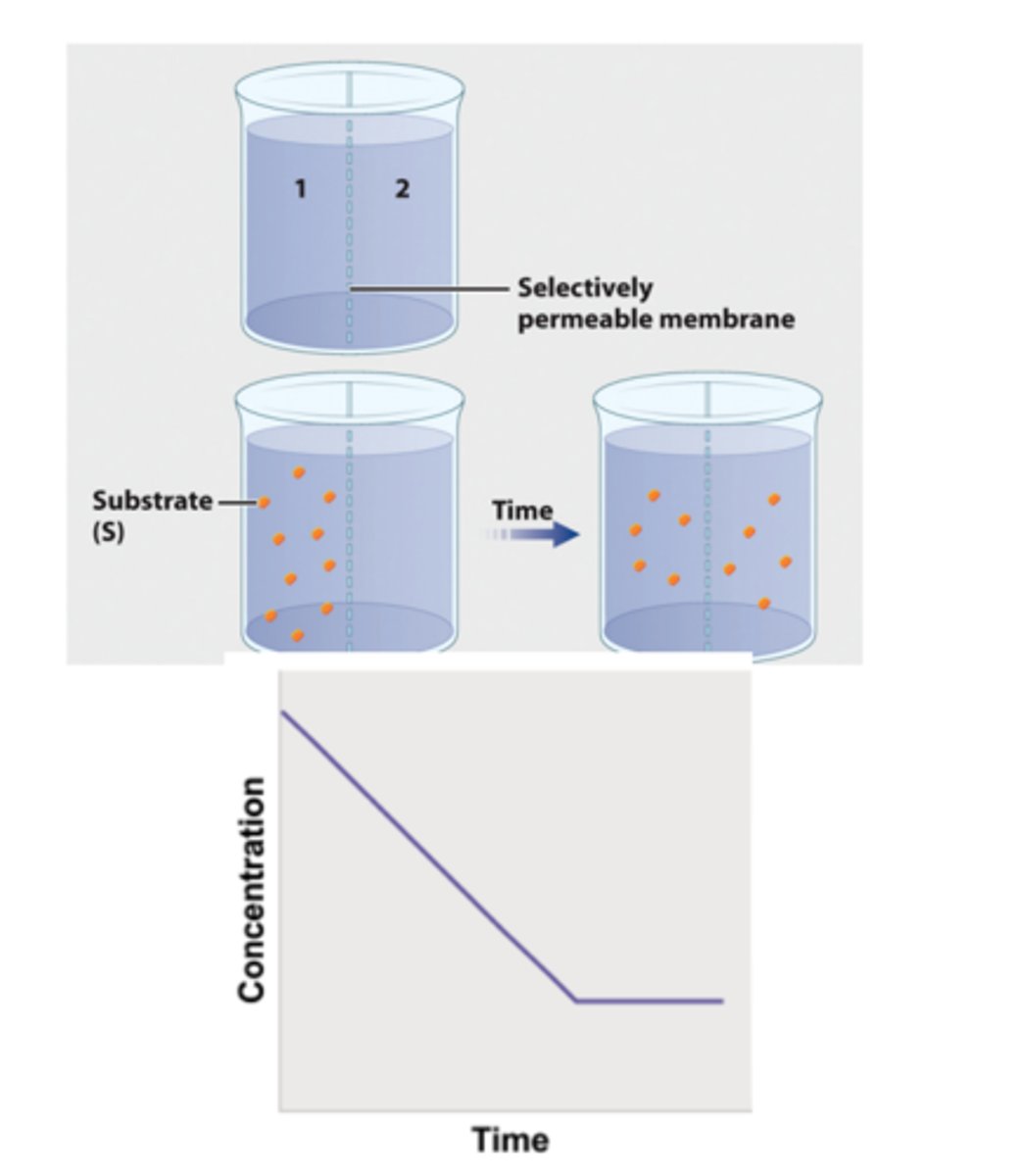
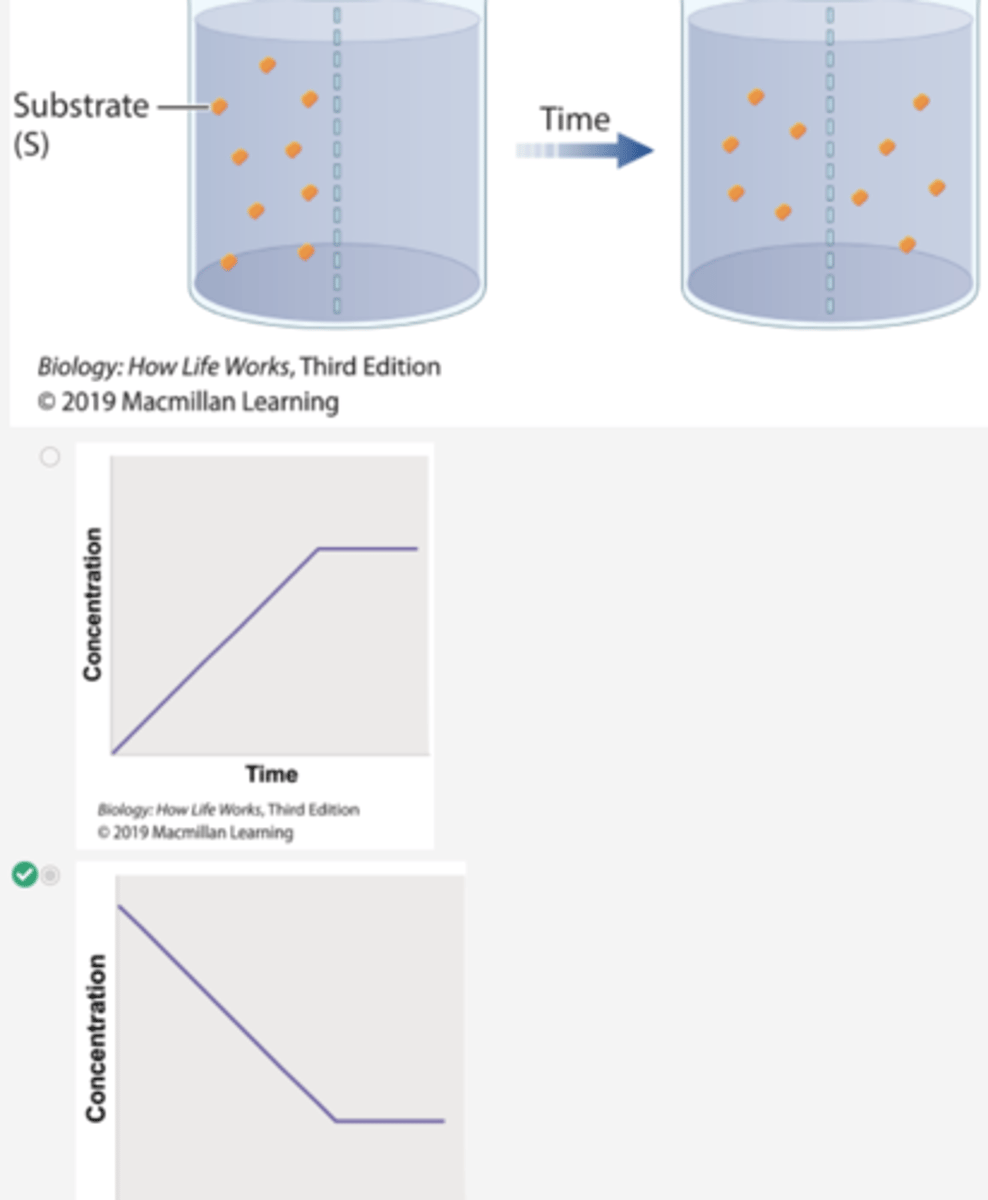
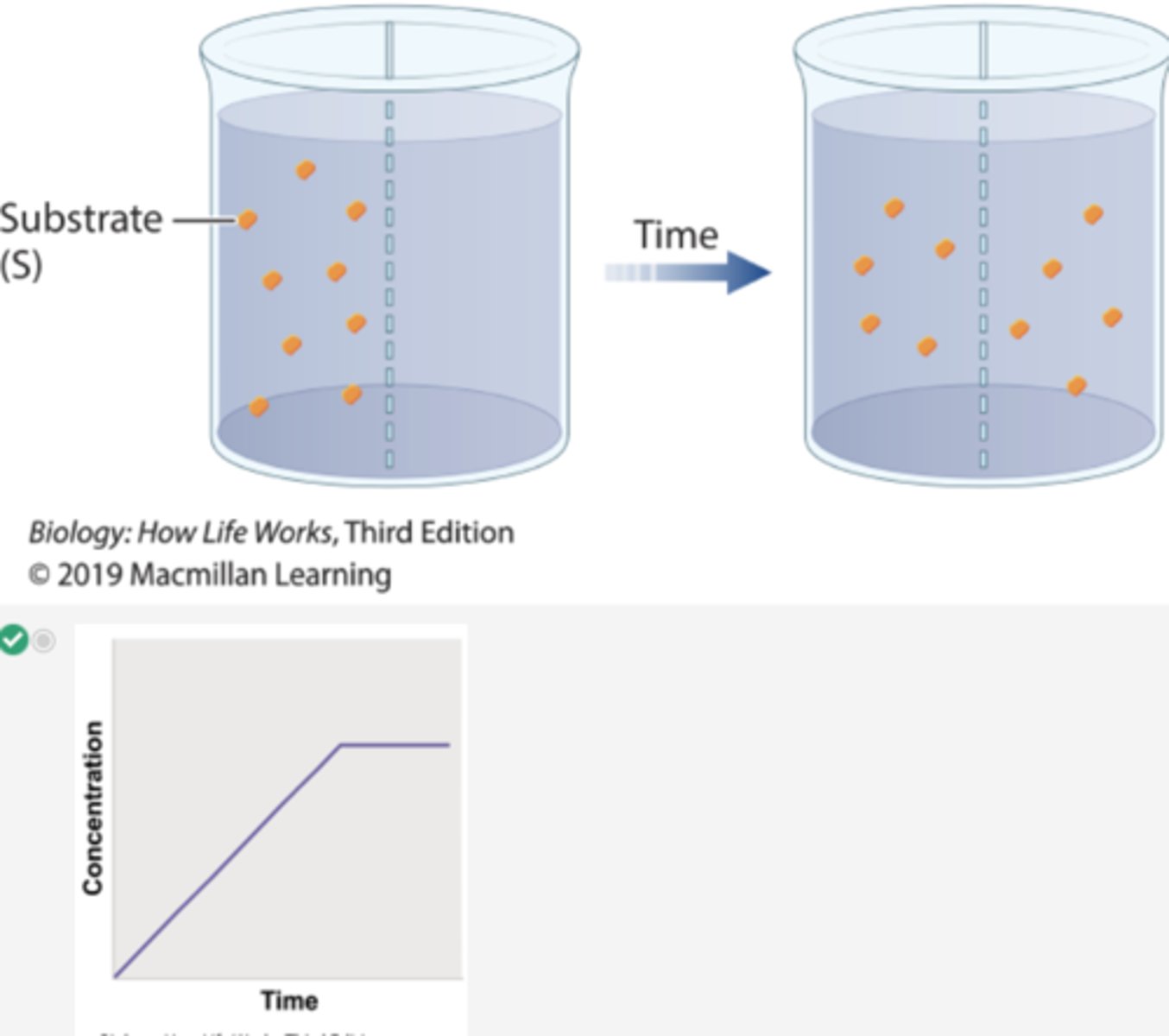
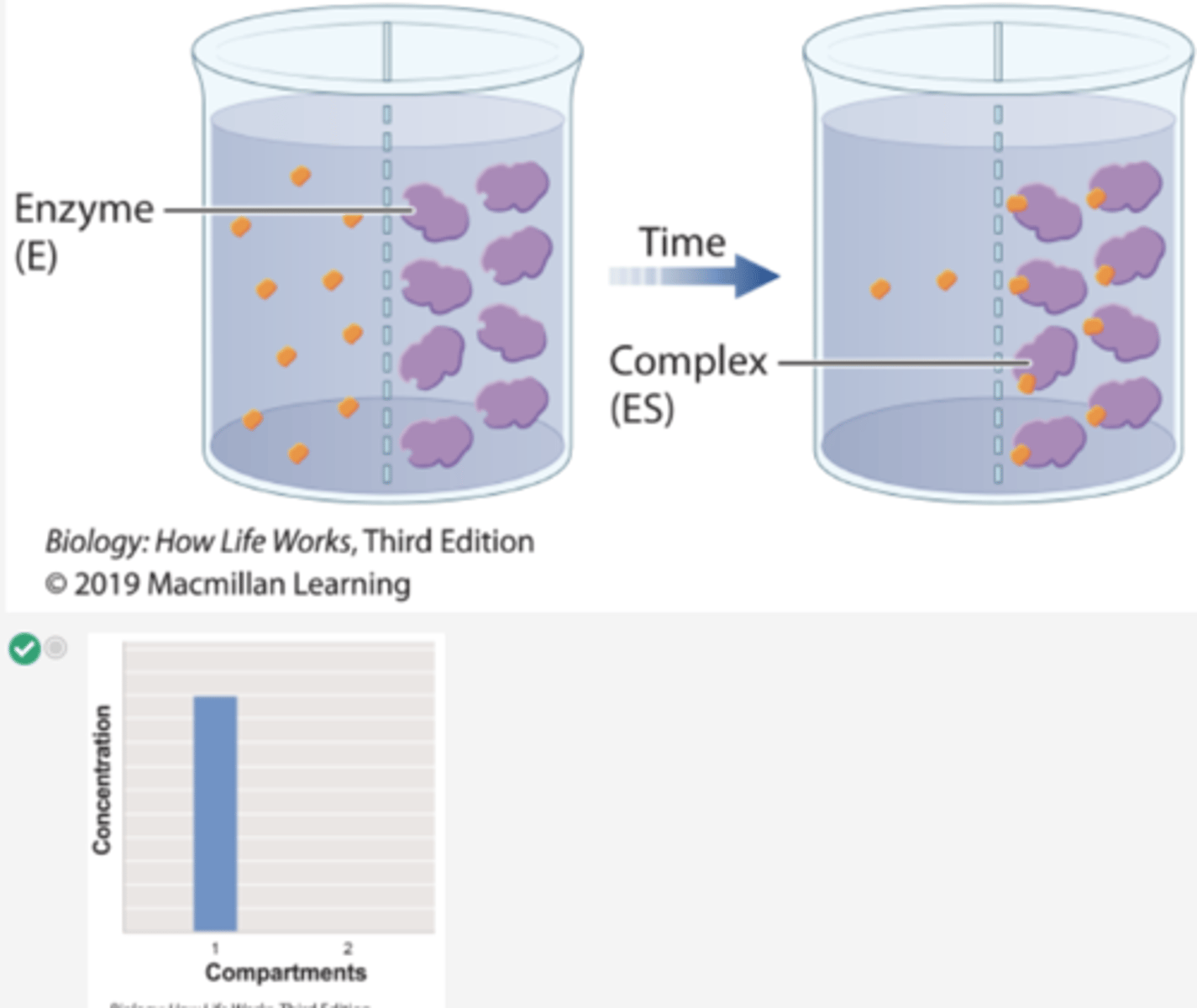
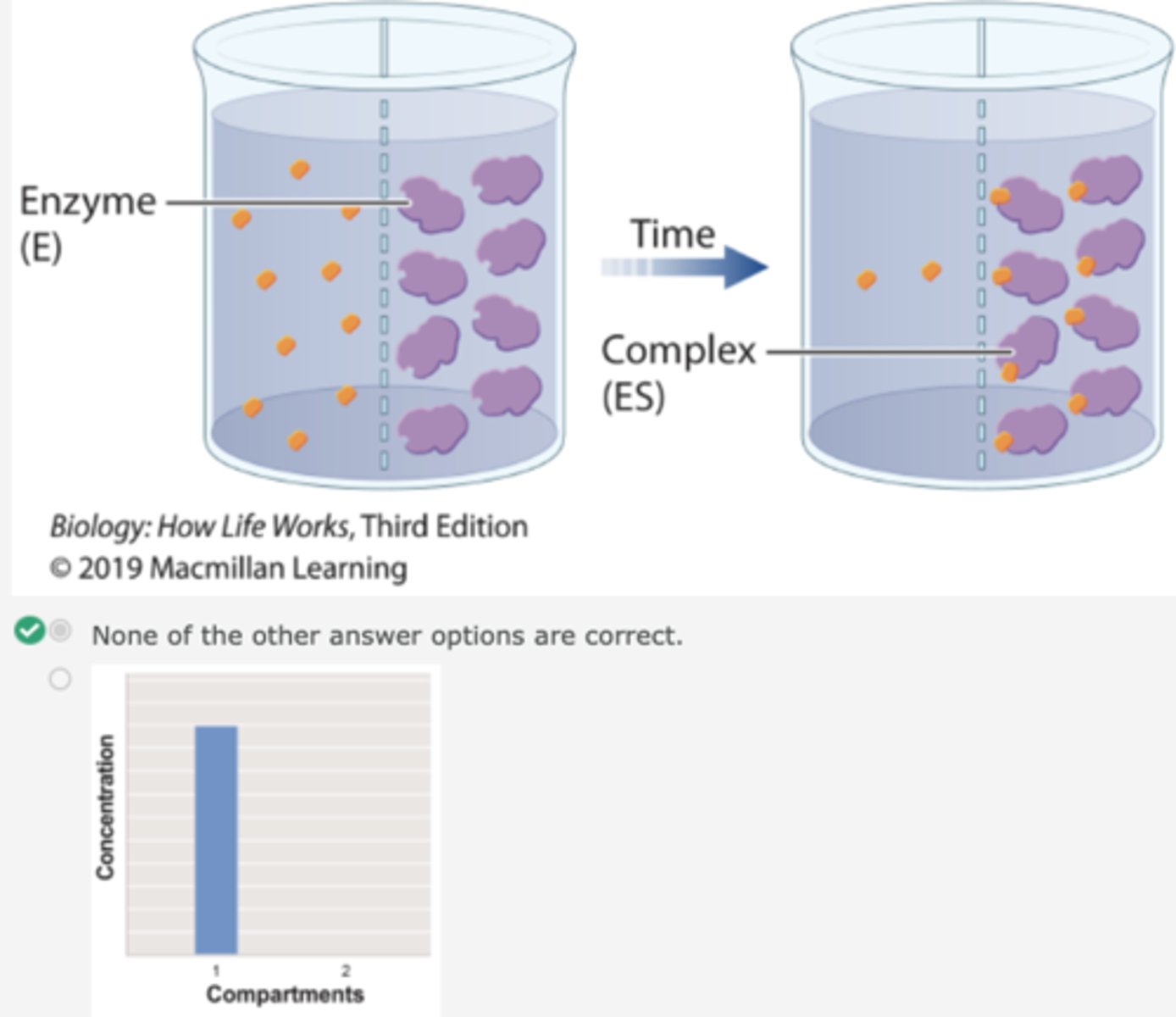
1. To show the formation of an enzyme/substrate complex, a container is separated into two compartments by a semipermeable membrane.
2. The membrane is permeable to β-galactoside and βthiogalactoside, but not permeable to the enzyme.
3. In the first beaker, radioactively labeled βthiogalactoside is added to compartment 1, and its movement is followed by measuring the level of radioactivity in the two compartments.
4. Over time, the level of radioactivity becomes the same in the two compartments.
continued experiment 1
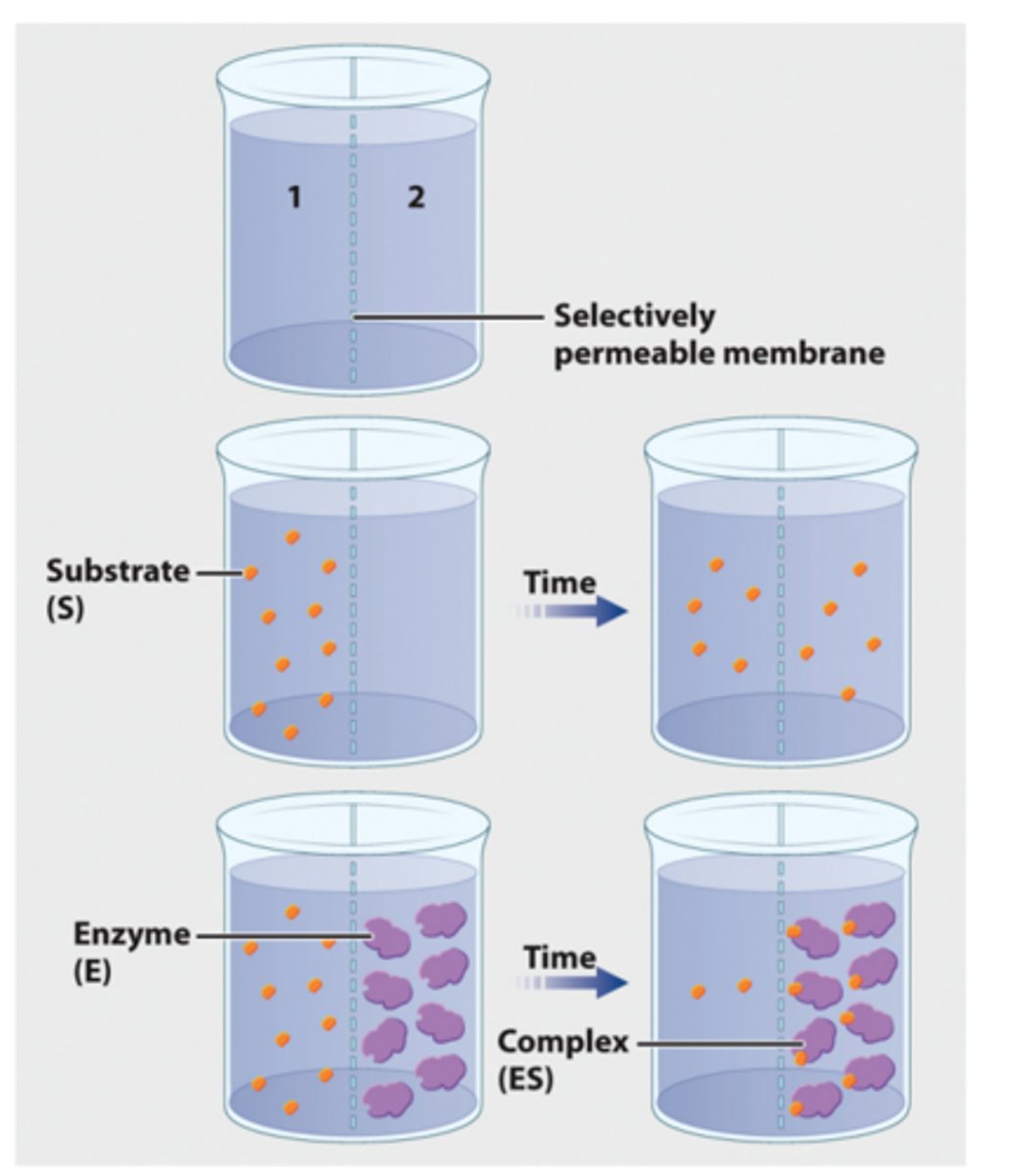
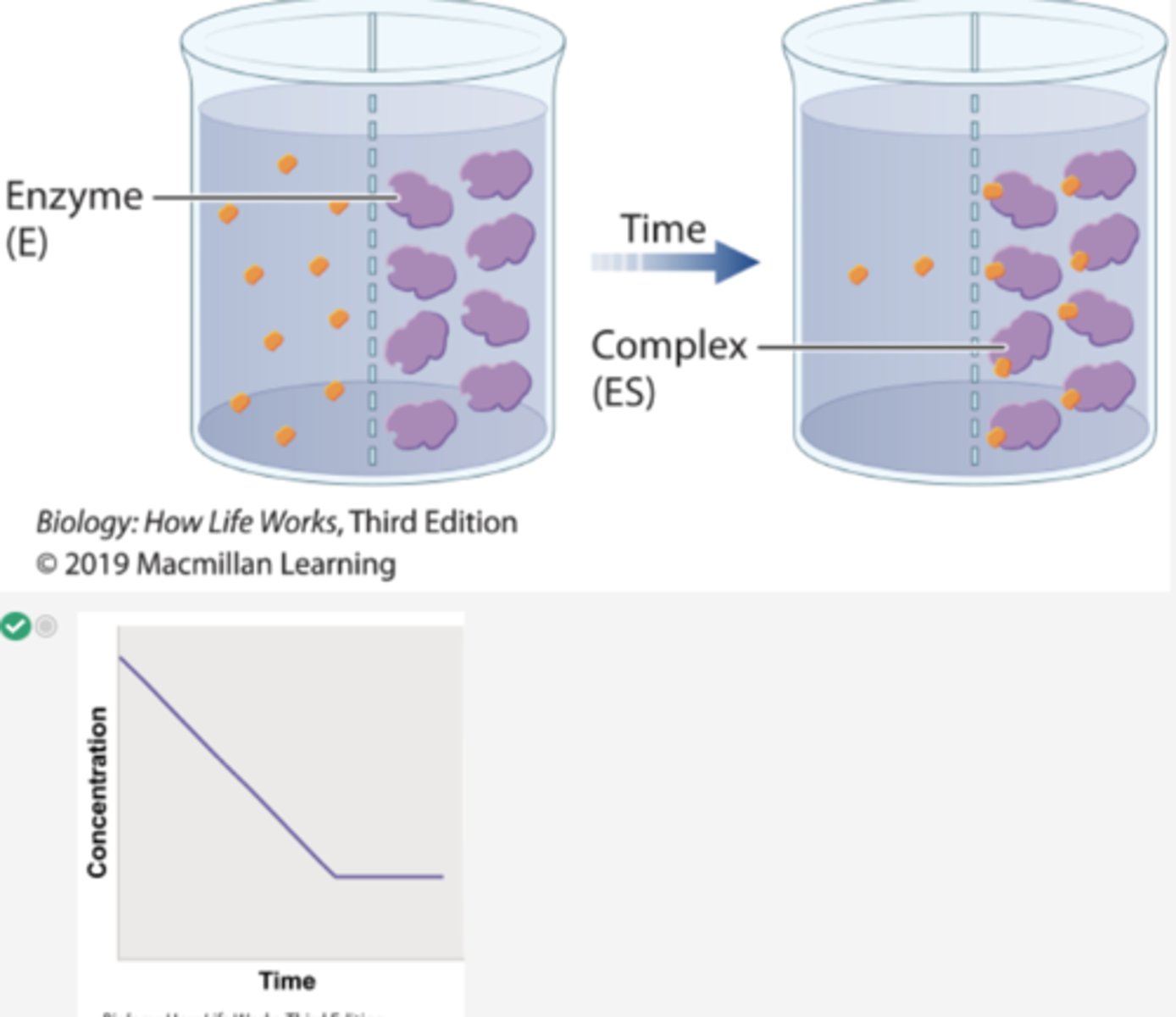
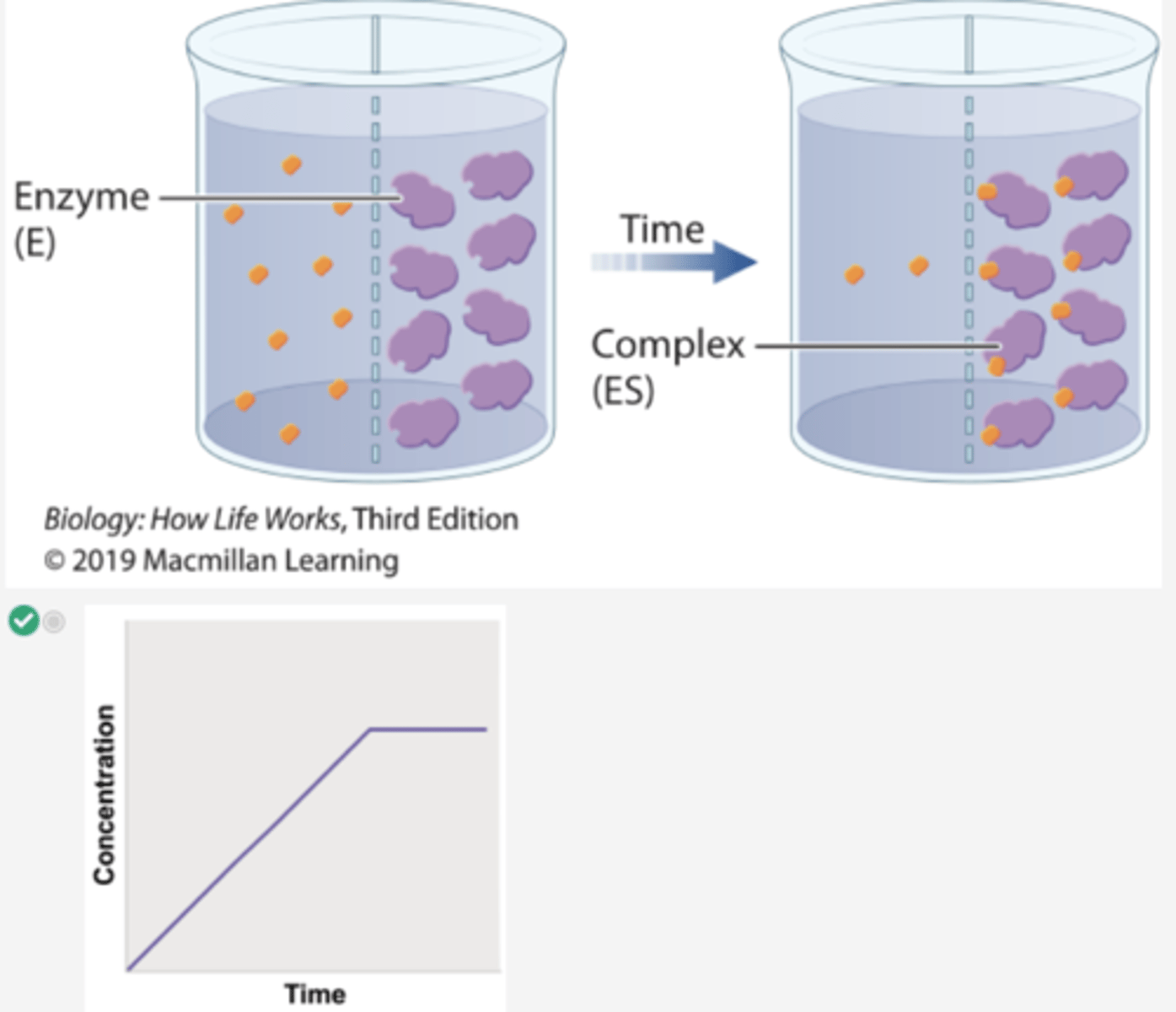
1. In a second experiment, radioactively labeled β-thiogalactoside is added to compartment 1 and enzyme is added to compartment 2.
2. Over time, the level of radioactivity is greater in compartment 2 than in compartment 1.
3. These results can be explained if the substrate diffuses from compartment 1 to compartment 2, forms a complex with the enzyme, and is not released.
4. In other words, the β-thiogalactoside and the enzyme form a complex.
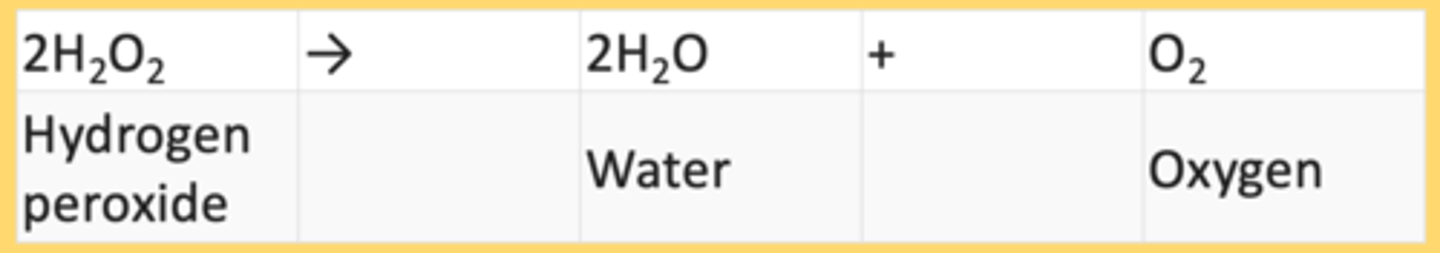
In a related experiment, scientists studied catalase, which is an enzyme that catalyzes the reaction:
In this experiment, we would expect an ES complex to be formed between:
catalase and hydrogen peroxide
(enzyme) + (substrate) = ES complex
kinetic energy
energy of motion
potenital energy
stored energy
-depends on position of object relative to its surrounding or on objects structure
example of potential energy
cell membrane can block movement of ions from 1 side to other, storing potential energy that is released if transmembrane chanel is opened
how does food contain energy?
-organic molecules (carbohydrates, fats, proteins) contains chemical energy (form of potential energy)
-carbon-carbon bonds & carbon-hydrogen bonds
-these covalent bonds weak which require a lot of energy to stay intact, so organic molecules store large amount potential energy due to their chemical structure
which of choices is an example of kinetic energy?
photon of light
which of choices is an example of potential energy?
an electrochemical gradient across the cell membrane
many cellular processes represent work that requires expenditure energy. Which of the actions is not a cellular process that requires the cell to expend energy?
diffusion of water into cells
chemical reactions part 2
-involves conversion reactants to products
-proceed through transition state that has large amount of energy
transition state
intermediate, highly unstable stage between two reactants & products in which old bonds are breaking & new ones are forming
what does a "energy barrier" represent?
difference in energy between the transition state and reactions= activation energy
enzymes
in cell, energy barrier is reduced by chemical catalysts ______.
late chemical energy is inversely related to height of energy barrier:
higher energy barrier, slower reaction
lower energy barrier, faster reaction
how do enzymes accelerate chemical reactions?
by reducing activation energy of reaction
The transition state is that portion of the progress of a chemical reaction with a large amount of energy found in _______ reactions.
both endergonic and exergonic
In this figure the solid blue curve plots free energy of a reaction against progress of that reaction. This reaction is:
endergonic
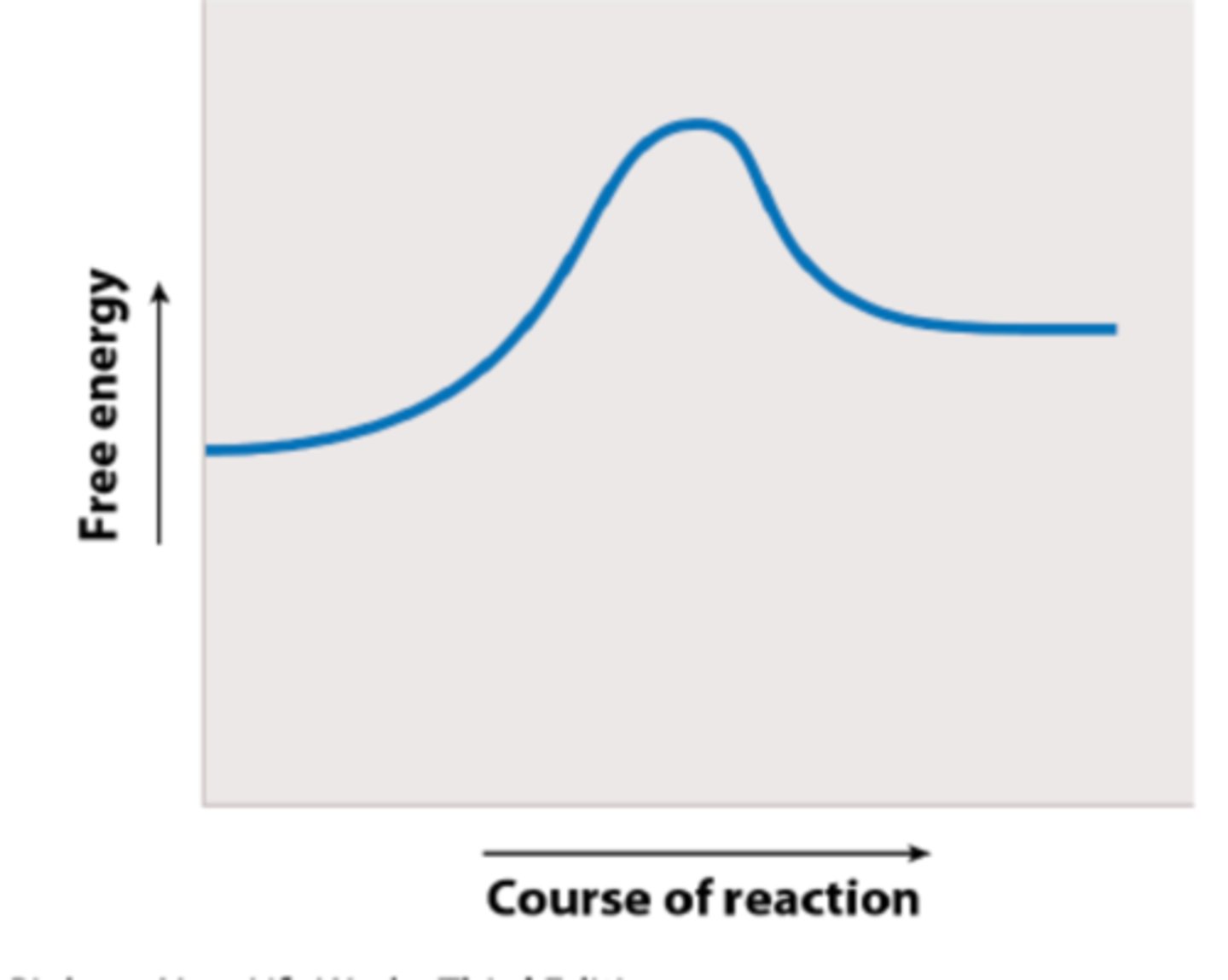
Which labeled arrow in the figure represents the activation energy (EA)?
arrow B
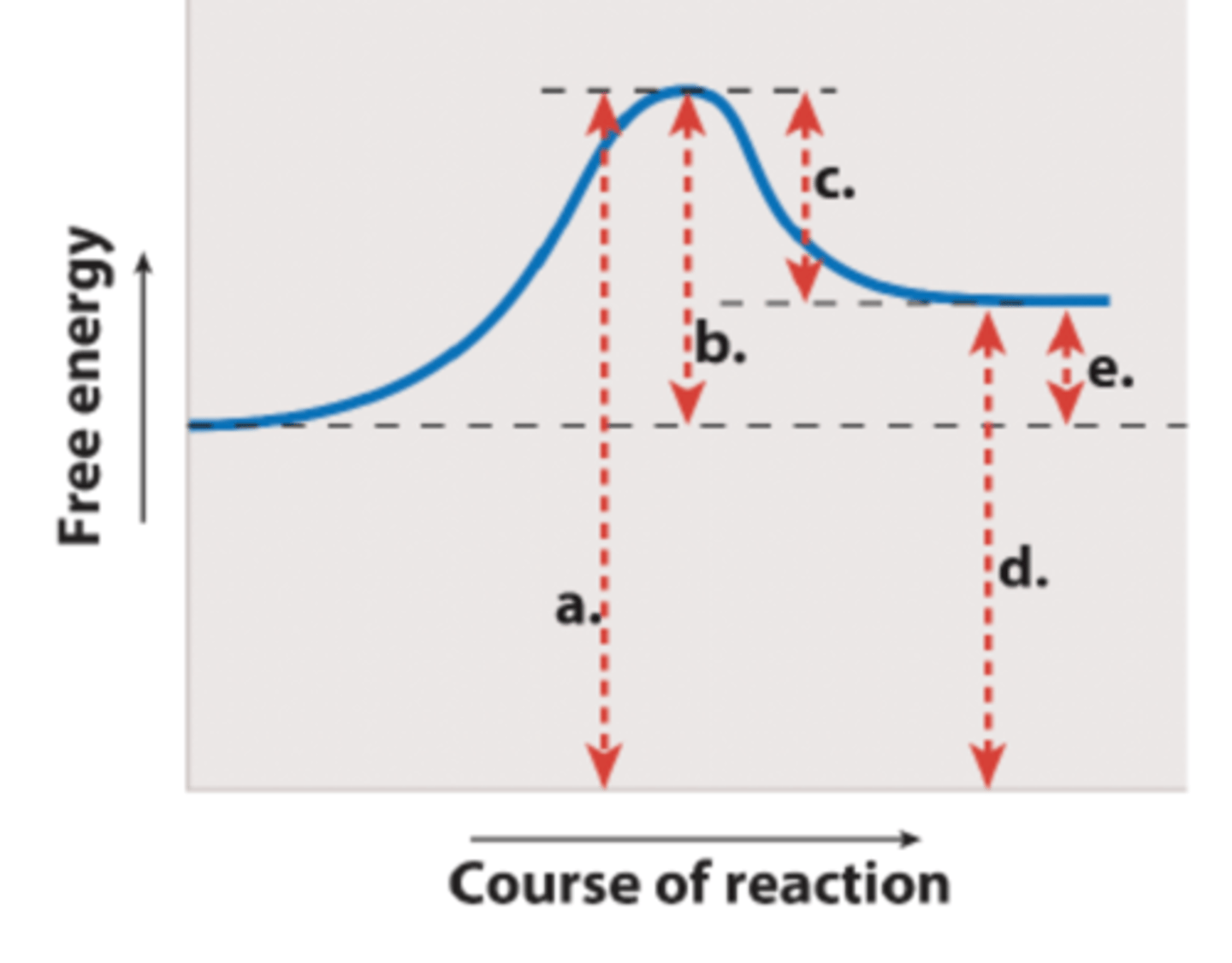
Which labeled arrow in the figure represents the change in free energy of the reaction (G)?
arrow E
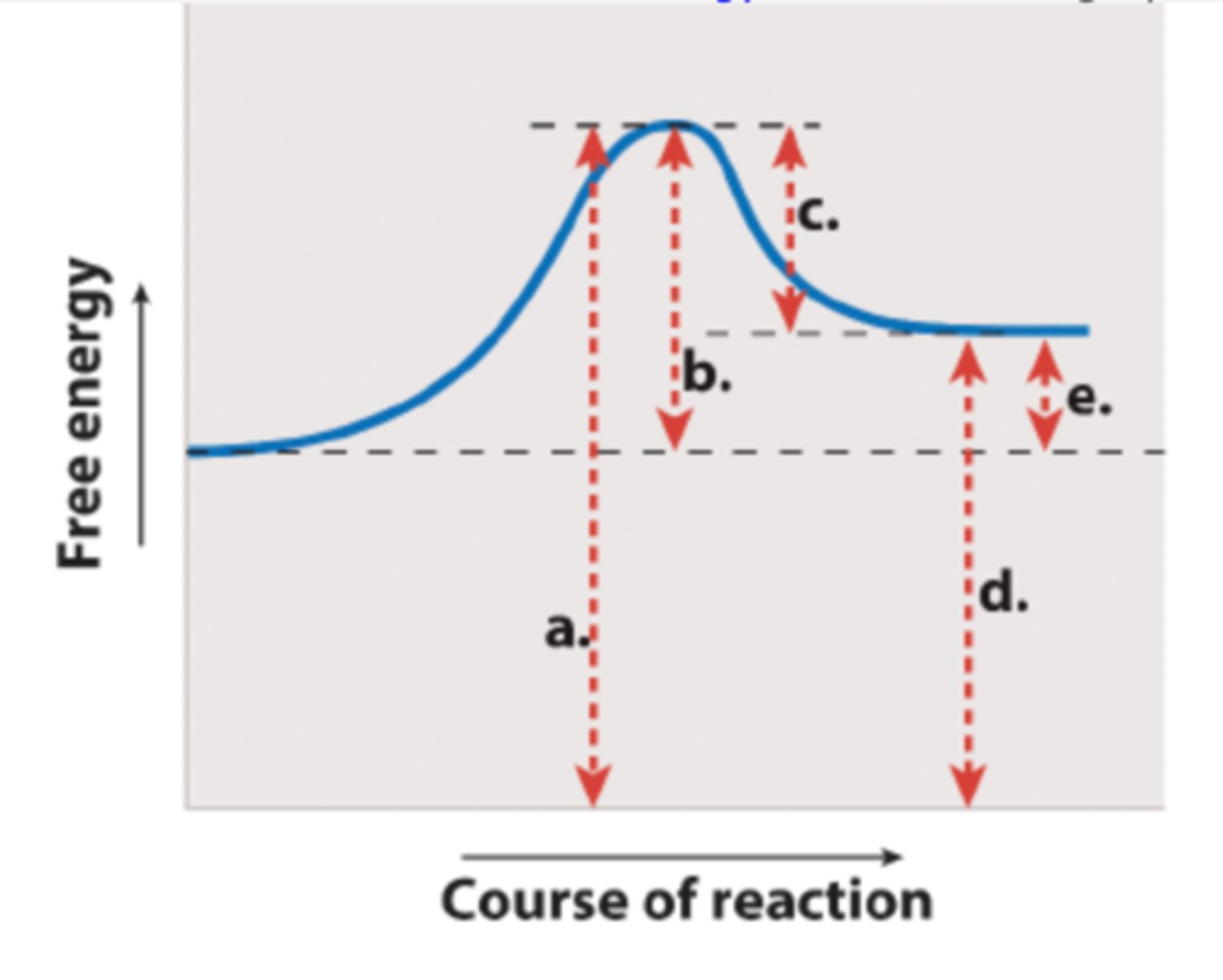
If you added an enzyme that catalyzes the reaction shown, you would predict that ____ would be reduced but ____ would remain the same.
arrow b; arrow e

enzyme activity is regulated. BUT,
Enzymes in cells are not always active.
-When the products of a type of enzyme's reaction build up or are not needed in the cell, those enzymes are "turned off" by inhibitors.
-When their products are needed and their reactants are present, those enzymes are "turned on" by activators.
-Cells use several different strategies to accomplish enzyme regulation.
-Enzyme inhibitors are used clinically to treat many disorders
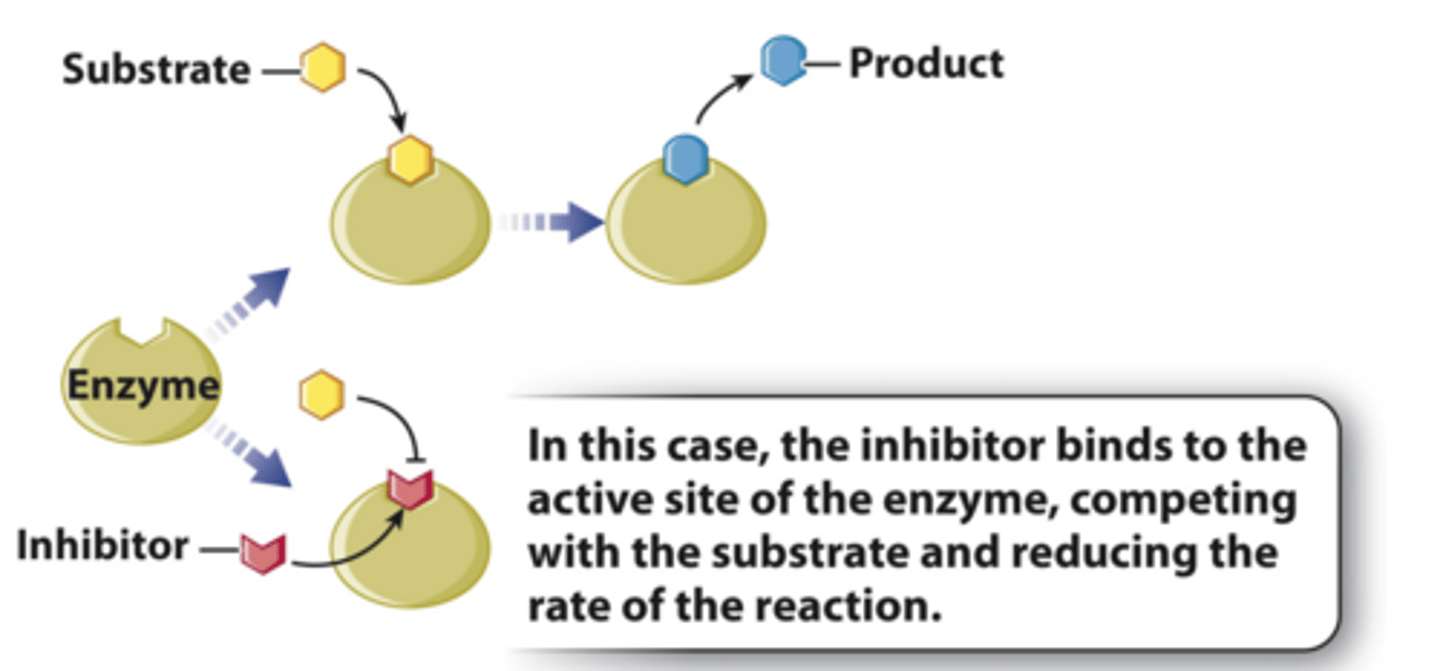
in cells, _____ function by binding to the enzyme active site
competitive inhibitors (noncovalent)
In cells, ___________ regulate enzymes by binding outside the active site.
allosteric regulators (noncovalent)
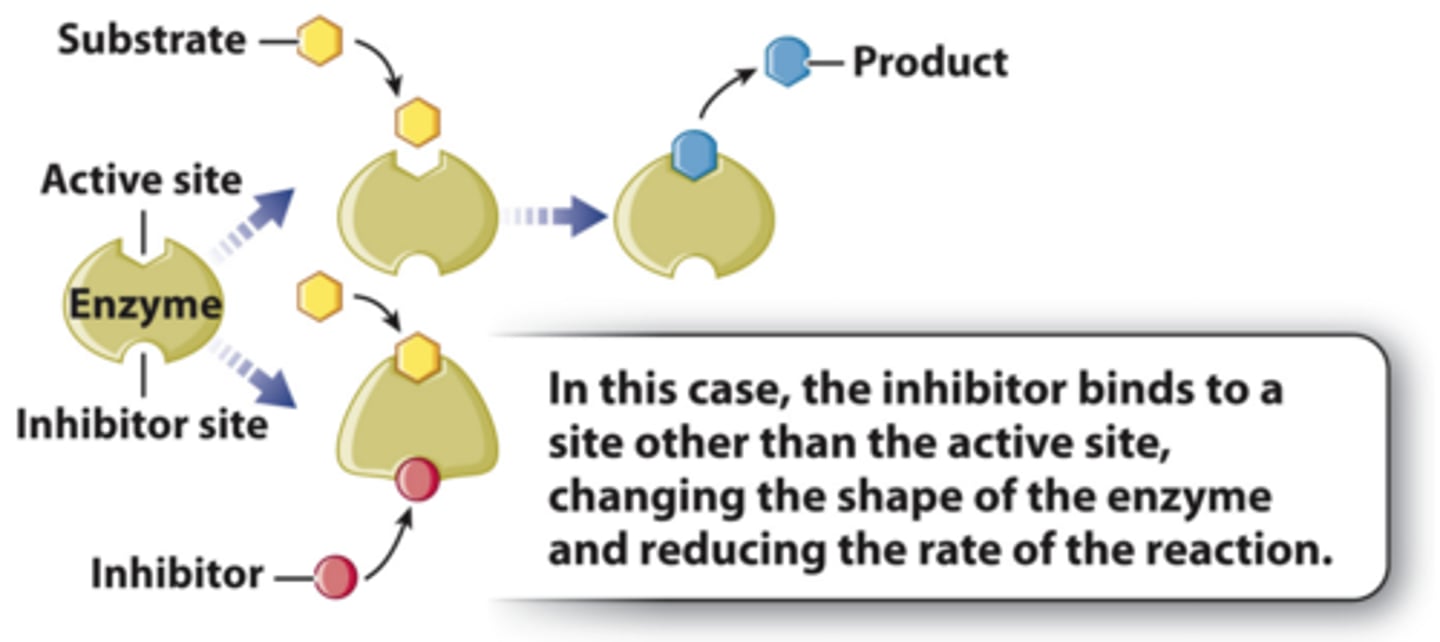
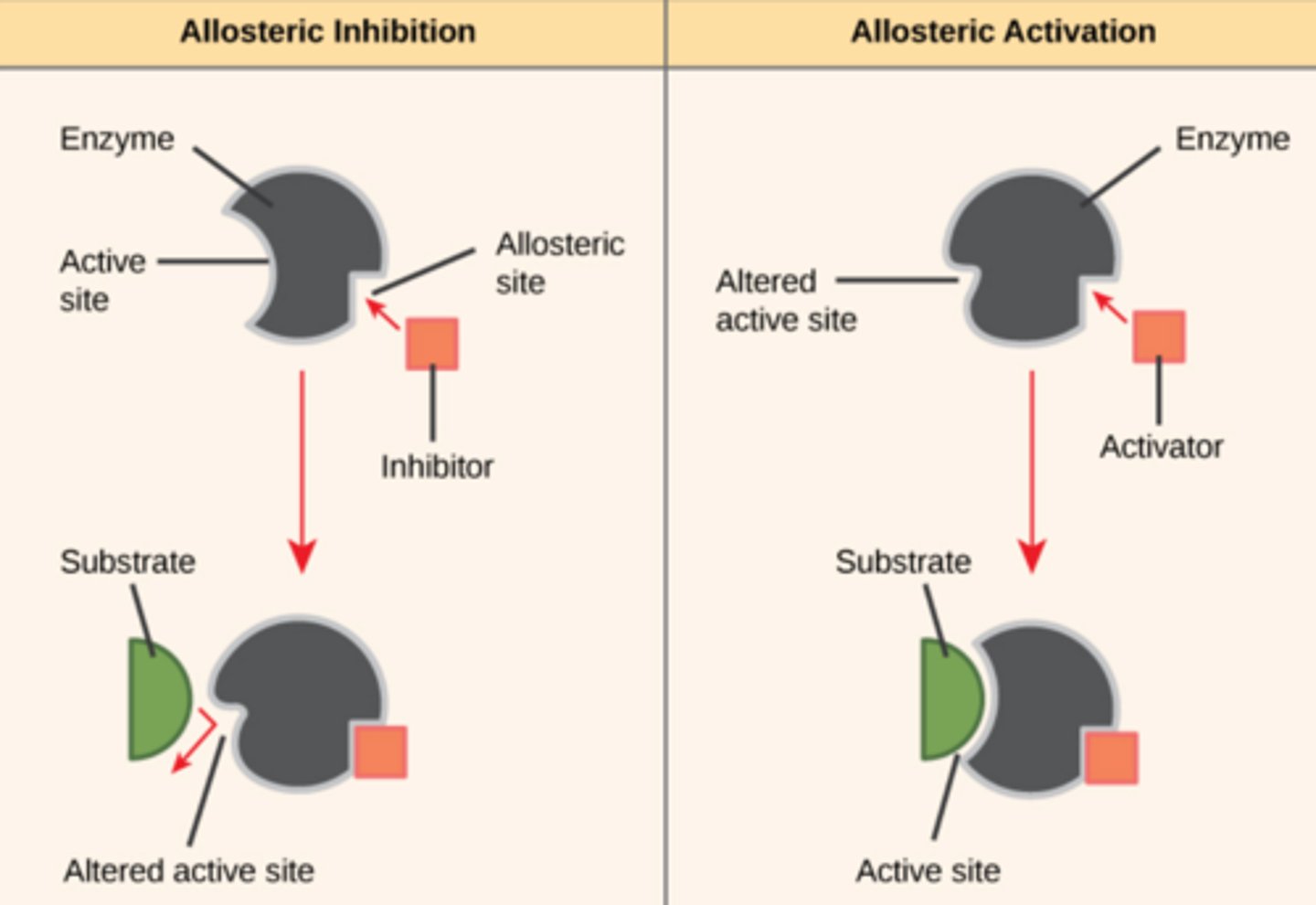
Allosteric Regulators Can be Activators or Inhibitors
Regulating enzymes: Covalent modification
-Reversible example: phosphorylation (adding a phosphate to a particular amino acid in an enzyme)
-Irreversible example: cleaving peptide bonds in the enzyme
Regulating Metabolic Pathway
1. In the synthesis of isoleucine from threonine, five reactions take place.
2. Once enough isoleucine is made, its production is shut down by the product isoleucine binding to the first enzyme in the pathway, threonine dehydratase.
3. The binding of isoleucine causes the enzyme's shape to shape, inhibiting its function.
4. The enzyme threonine dehydratase is an example of an allosteric enzyme, an enzyme that is activated or inhibited when binding to another molecule changes its shape.
5. The process in which the final product inhibits the first step of the reaction is known as negative feedback or feedback inhibition.
Which shows inhibition by an allosteric inhibitor?
C
What hypothesis did this experiment test?
Enzymes form complexes with substrates.
Before this experiment was performed, Kurt Stern had already shown that the enzyme catalase forms a complex with its substrate. Therefore, this current set of experiments:
investigated a different enzyme and substrate.
Which graph shows the concentration of the substrate β-thiogalactoside in compartment 1 over time in Experiment 1?
Which graph shows the concentration of the substrate β-thiogalactoside in compartment 2 over time in Experiment 1?
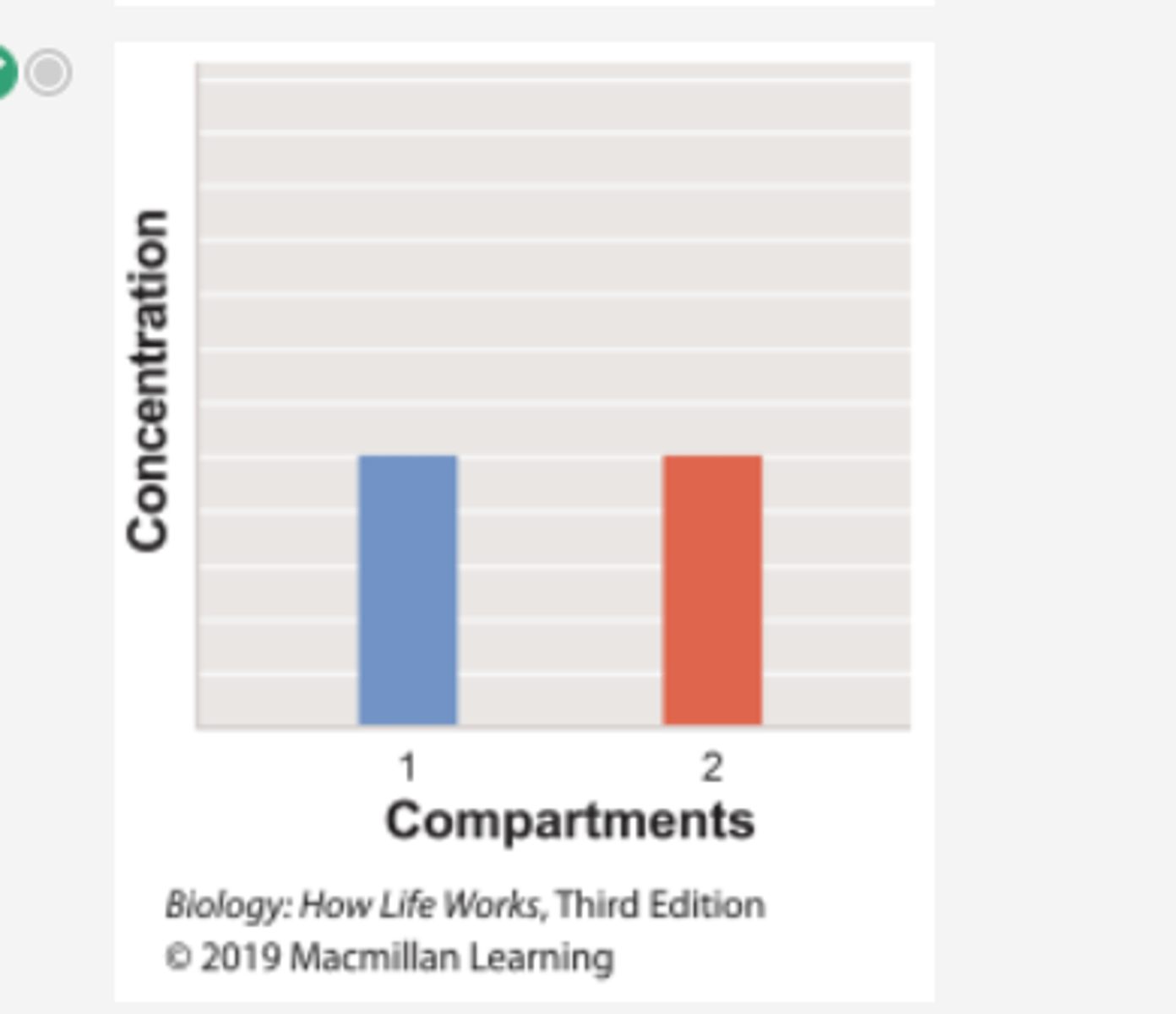
Which histogram shows the concentration of substrate β-thiogalactoside in compartments 1 and 2 at the start of Experiment 1?

Which histogram shows the concentration of substrate β-thiogalactoside in compartments 1 and 2 at the end of Experiment 1?
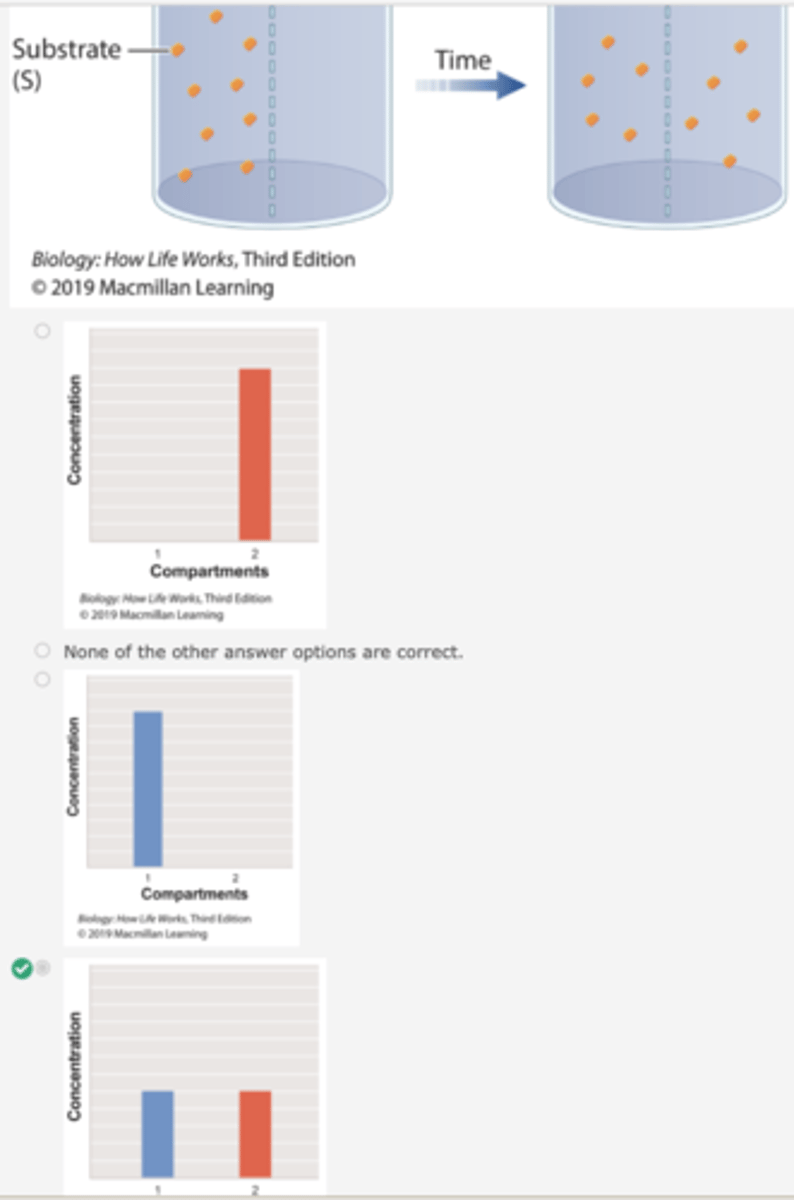
Which graph shows the concentration of substrate β-thiogalactoside in compartment 1 over time in Experiment 2?
Which graph shows the concentration of total substrate β-thiogalactoside in compartment 2 in Experiment 2?
Which histogram shows the concentration of substrate β-thiogalactoside in compartments 1 and 2 at the start of Experiment 2?
Which histogram shows the concentration of substrate β-thiogalactoside in compartments 1 and 2 at the end of Experiment 2?
In a related experiment, Kurt Stern studied catalase, which is an enzyme that catalyzes the reaction:
2H2O2→2H2O+O2
Hydrogen peroxide Water Oxygen
In this experiment, we would expect a complex to be formed between:
catalase and hydrogen peroxide.
Kurt Stern used spectral analysis to test whether the enzyme forms a complex with its substrate by shining a white light on the reaction as it took place. Different molecules absorb different wavelengths of light. The maximum absorption of a wavelength by each molecule is called the absorption peak. By analyzing the patterns of absorption peaks as the reaction proceeded, Stern could determine whether or not a complex was formed. What do you predict Stern observed as the reaction proceeded?
all of answers correct
If Experiment 1 were repeated using the substrate β-galactoside instead of β-thiogalactoside, which of the histograms would show the concentration of β-galactoside in compartments 1 and 2 at the end of the experiment?
Why regulate gene expression?
The regulation of gene expression conserves energy and space. It would require a significant amount of energy for an organism to express every gene at all times, so it is more energy efficient to turn on the genes only when they are required.
what gives cells identities?
type of genes from zygote that are expressed in each one, differential gene expression
although they share, different cell types look & function differently from one another why?
because each type of cell expresses different sets of genes. regulation of gene expression assures that the right genes are expressed at the right time
eukaryotic gene regulation can take place:
1. through chemical modification of chromatin or histones
2. during transcription
3. during RNA processing
4. during translation
the human body contains approximately 200 major cell types. they look & function differently from one another because each:
expresses a different set of genes
DNA wrapped around proteins called _______
histones
Epigenome
-DNA & histones covered with chemical tags
-these tags react to signals from outside world (diet/stress)
-it adjusts specific genes in our genomic landscape in response to changing environment
Epigenome details:
-shapes physical structure of genome
-tightly wraps inactive genes, making them unreadable
-relaxes active genes, making them easily accessible
-flexible
Epigenetic- over and above inheritance
1. Epigenetic effects are due changes that do not change the DNA sequence but do affect gene expression
2. These changes sometimes cause changes in gene expression.
3. They can be inherited, but are often reversible and responsive to changes in the environment.
4. Epigenetic changes include chromatin remodeling, histone modifications, and DNA methylation