Biological molecules - Proteins and enzymes
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
What are proteins polymers of?
amino acids
What are proteins made of?
long chains of amino acids
How is a dipeptide formed?
By the condensation of two amino acids
What does a condensation bond between 2 amino acids form?
A peptide bond
How are polypeptides formed?
By the condensation of many amino acids.
What does a functional protein contain?
one or more polypeptides
What does an amino acid contain?
A central carbon atom, Carboxylic group, amino group, H atom and R group (determines type of amino acid)
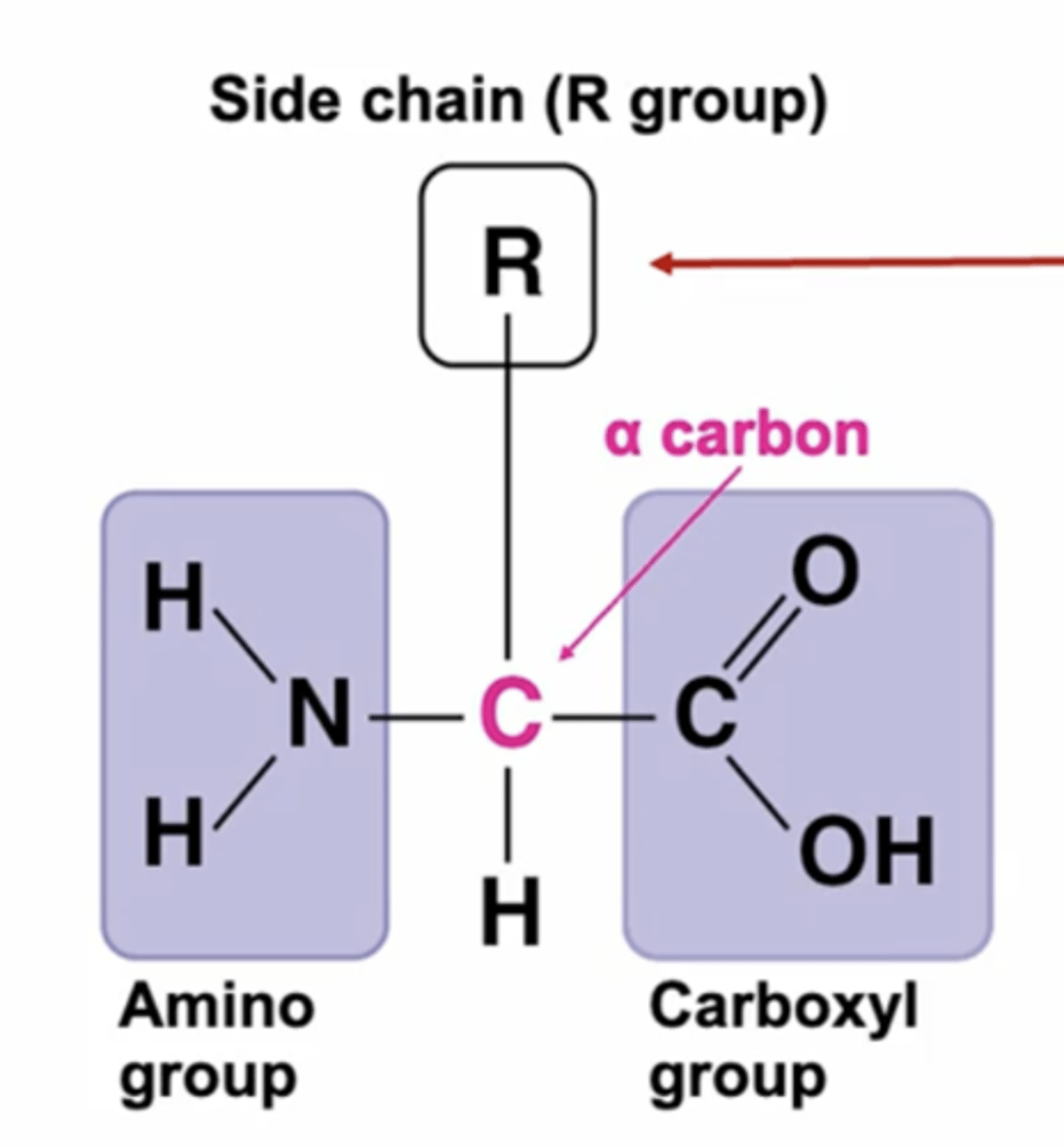
What is the carboxyl group of amino acids?
COOH
What is the amine group in an amino acid?
NH2
What does the R group in an amino acid represent?
The variable side chain
How do the 20 amino acids differ?
different R groups
How many nucleotides code for an amino acid?
3
What is the primary structure of a protein?
sequence of amino acids in a polypeptide chain
What does the primary structure of a protein determine?
It's ultimate shape, therefore it's function
What does the secondary structure of a protein determine?
The shape that the chain of amino acids make
What are the two types of secondary structures in proteins?
Alpha helix and beta pleated sheet
What is the secondary structure of proteins formed by?
Hydrogen bonding between peptide bonds
How does hydrogen bonding affect the secondary structure?
causes the polypeptide chain to coil into either an alpha-helix or a beta-pleated sheet structure
Describe how the structure of a protein depends on the amino acids it contains. (5 MARKS)
- Structure is determined by (relative) position of amino acid/R group/interactions
- Primary structure is sequence/order of amino acids
- Secondary structure formed by hydrogen bonding (between amino acids)
- Tertiary structure formed by interactions (between R groups)
- Creates active site in enzymes
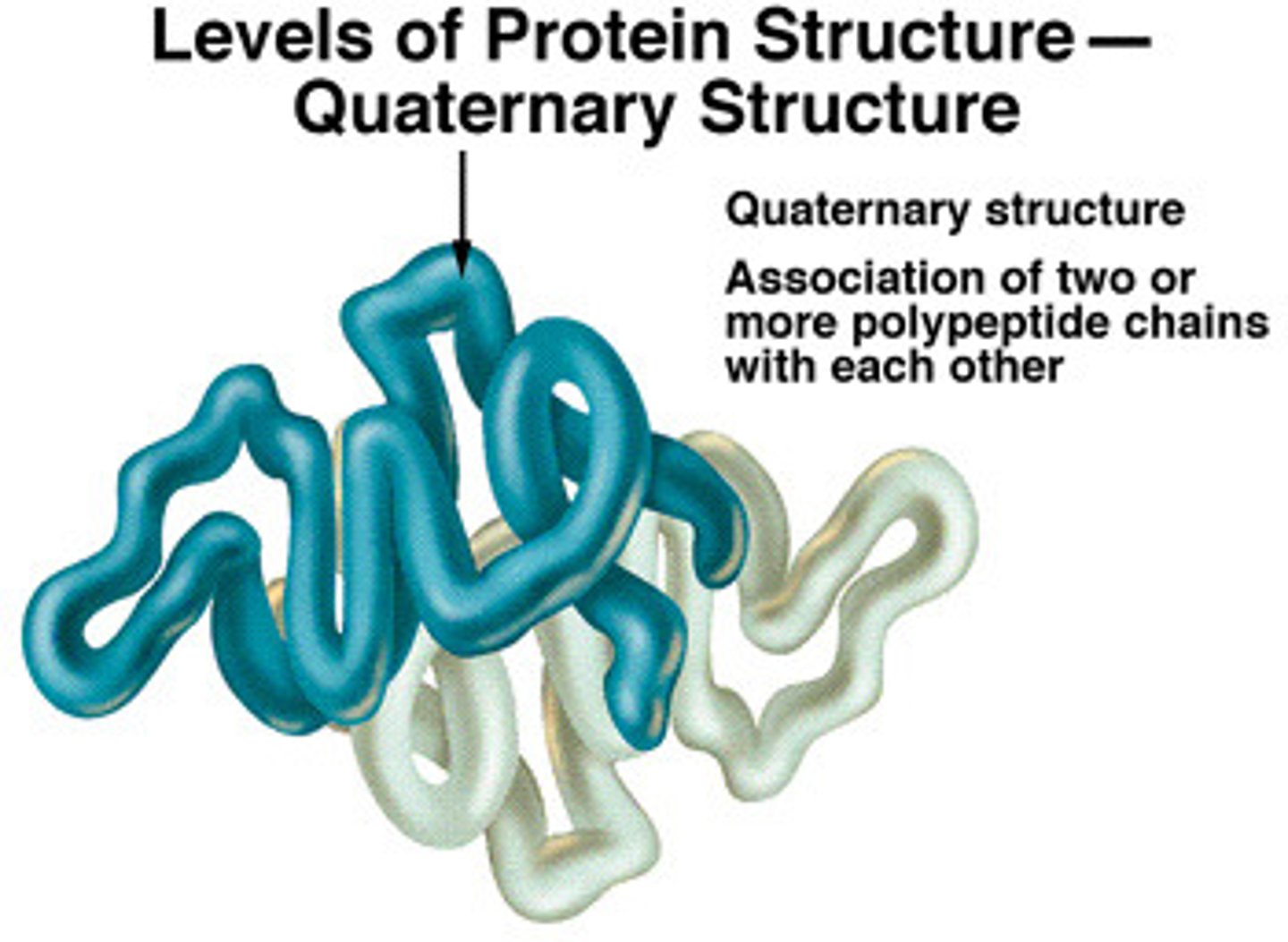
- Quaternary structure formed by interactions/bonds between polypeptides
Describe how amino acids join to form a polypeptide so there is always NH2 at one end and COOH at the other end.
One NH2 group joins to a COOH group to form a peptide bond, so there is a free NH2 group at one end and a free COOH group at the other as each amino acid is facing the same direction in the chain.
The secondary structure of a polypeptide is produced by bonds between amino acids. Describe how.
Hydrogen bonds between NH group and C=O group, forming beta pleated sheets or an alpha helix
Two proteins have the same number and type of amino acids but different tertiary structures. Explain why.
Different sequence of amino acids / Different primary structure so forms ionic / hydrogen / disulfide bonds in different places
What is the tertiary structure of a protein?
3D structure formed from further twisting and folding
What types of bonds maintain the tertiary structure of proteins?
Disulfide bridges, ionic bonds, and hydrogen bonds
What are disulfide bridges in proteins?
Interactions between the sulfur in the R group of the amino acid cysteine
Where do disulfide bridges form?
between R groups that contain sulphur e.g. cysteine
Two proteins have the same number and type of amino acids but different tertiary structures. Explain why.
Different sequence of amino acids so forms ionic / hydrogen / disulfide bonds in different places
How do ionic bonds in proteins differ from disulfide bridges?
Ionic bonds are weaker and easily broken by pH changes
Where do ionic bonds in the tertiary structure form?
between positive and negative R groups
Describe hydrogen bonds in the tertiary structure.
Numerous but easily broken
Describe the tertiary structure of a protein. (3 marks)
- The further folding of the secondary structure
- To create a unique 3D structure
- Held in place by hydrogen, ionic, and disulfide bonds.
What is the quarternary structure of a protein?
the structure of the protein when multiple polypeptide chains are linked (in various ways)
Describe the test for proteins (in a food sample).
Add biuret reagent
If positive - turns purple
If negative - stays blue
What are some functions of proteins?
form muscles, transport O2, and act as hormones and enzymes
What roles do proteins play in the body?
Enzymes, Antibodies, Transport and structural proteins
What are enzymes?
biological catalysts
How do enzymes increase rate of reaction?
by lowering the activation energy
What is the active site of an enzyme?
where it binds its substrate
Describe the induced fit model of an enzyme
Substrate binds to the active site and active site changes shape (slightly) so it is complementary to substrate
Formation of an enzyme-substrate complex increases the rate of reaction. Explain how.
Reduces activation energy due to bending bonds
Name 5 factors that affect the rate of enzyme-controlled reactions.
- Enzyme concentration
- Substrate concentration
- Concentration of inhibitors
- pH
- temperature
What is the active site determined by?
tertiary structure
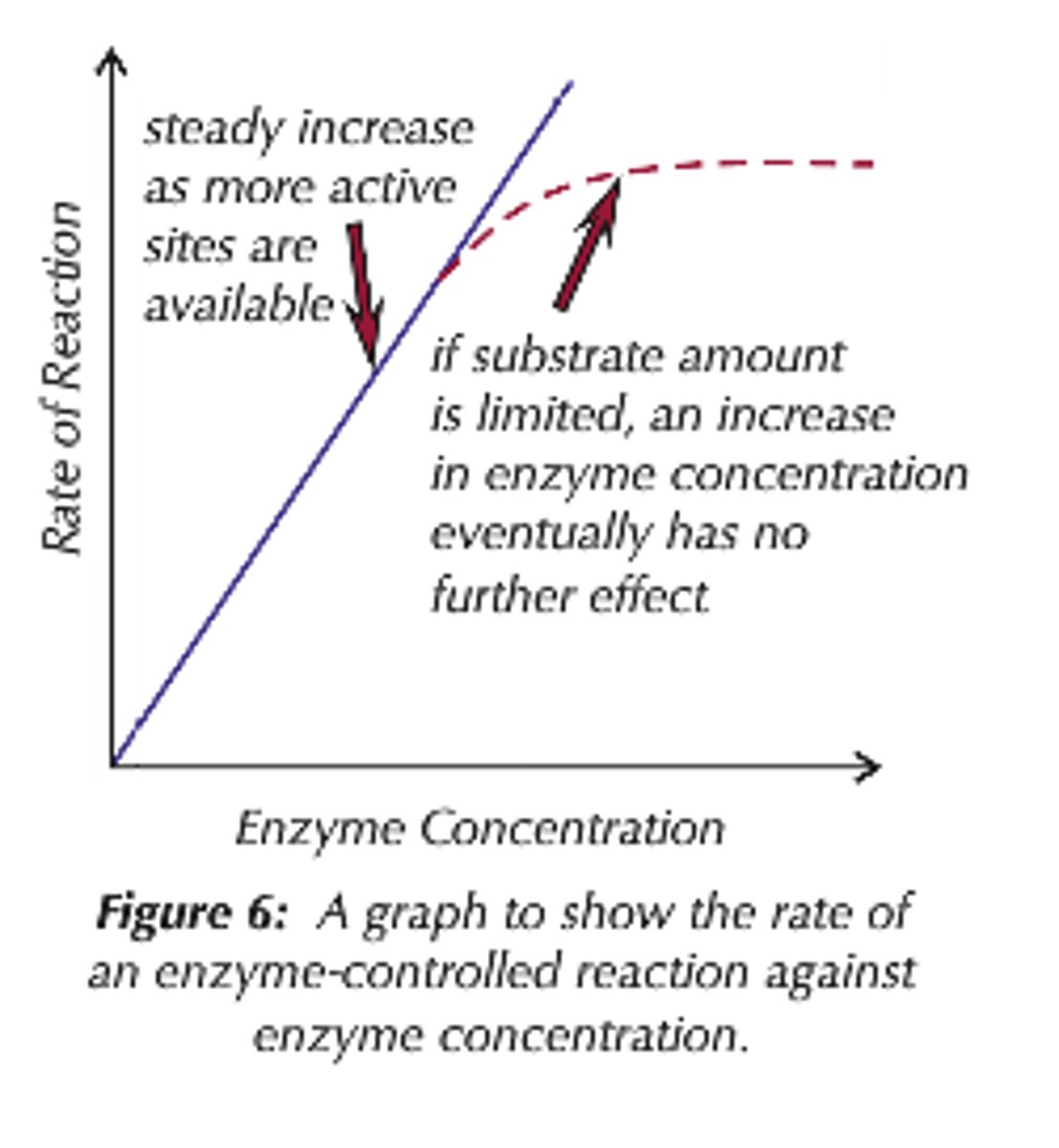
How does enzyme concentration affect rate of reaction?
The rate of reaction increases then plateaus as substrate concentration becomes the limiting factor.
How does substrate concentration affect rate of reaction?
Rate of reaction increases then plateaus as enzyme concentration becomes the limiting factor
How does pH affect rate of reaction?
Enzymes have a narrow optimum pH range. Outside range = denaturation.
How does temperature affect rate of reaction?
increases rate of reaction because increased temperature increases speed of the reacting particles so they collide more frequently and more energetically
What formula is used to calculate the pH using the hydrogen ion concentration of a solution?
−log₁₀(H+)
How does above optimum temperature affect enzyme activity?
Denatures enzymes, decreasing rate of reaction.
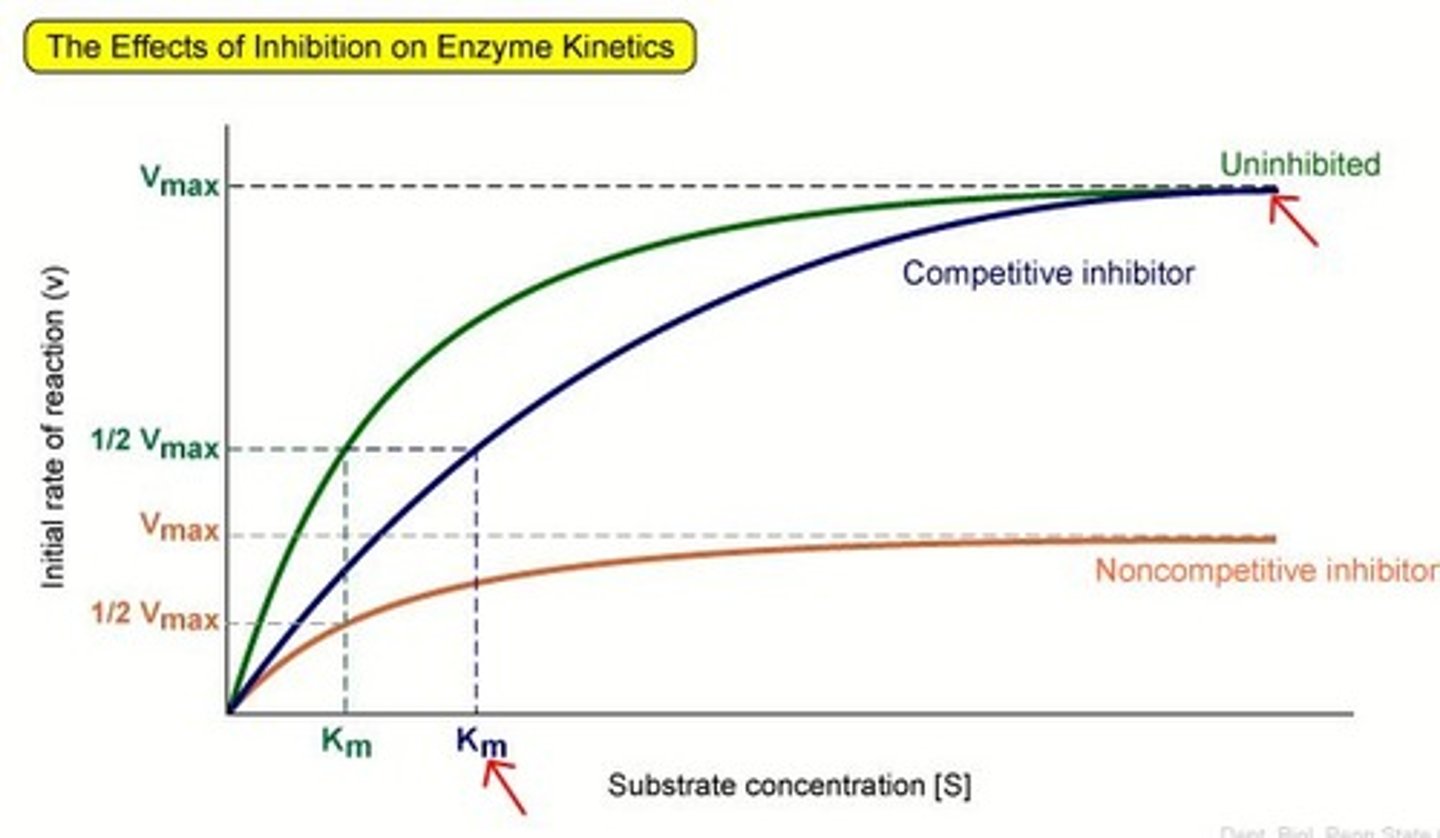
What effect do competitive reversible inhibitors have on rate of reaction?
decrease rate of reaction by blocking active sites
How do non-competitive reversible inhibitors affect rate of reaction?
decrease rate of reaction by altering the enzyme's shape
Explain how a change in a sequence of DNA bases could result in a non-functional enzyme. (3 marks)
- Change in sequence of amino acids - primary structure
- Change in bonds leads to change in tertiary structure / active site (of enzyme)
- Substrate cannot bind and no enzyme-substrate complexes form
Enzymes are extremely important in order to increase rates of reaction.
Describe how this happens. (4 marks)
Enzymes lower the activation energy, by binding to substrates and allowing bonds to be broken and new bonds to form.