Bonding and Structure
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
Ionic bonding
the strong electrostatic force of attraction between oppositely charged ions
Effects of ionic radius and ionic charge on the strength of ionic bonding
charge on the ions is the most important factor
The higher the charge, the stronger the ionic bonding
The smaller the ionic radius, the stronger the ionic bonding
Formation of ions
anions gain electrons
Cations lose electrons
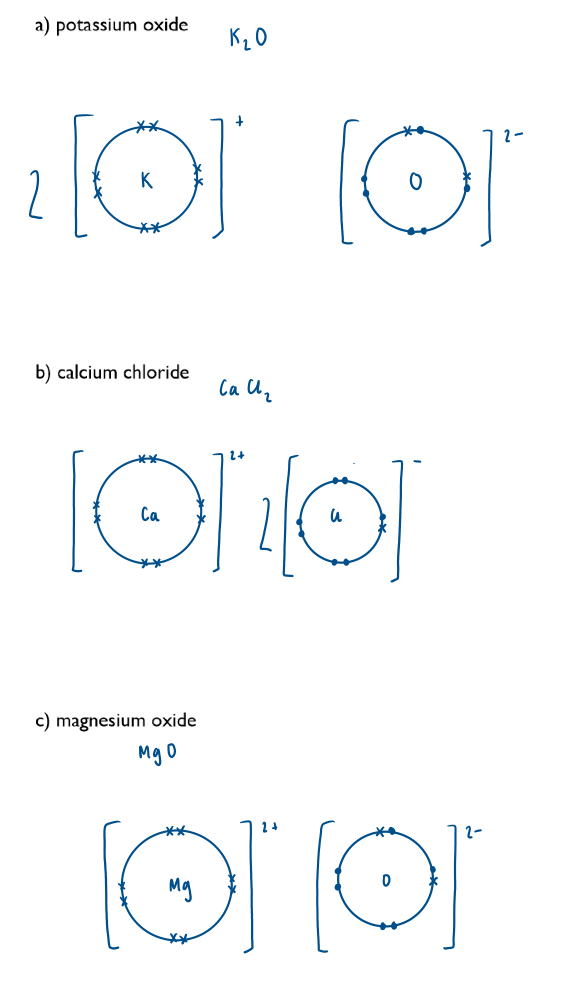
Draw electronic configuration diagrams of cations and anions using dot-and-cross diagrams
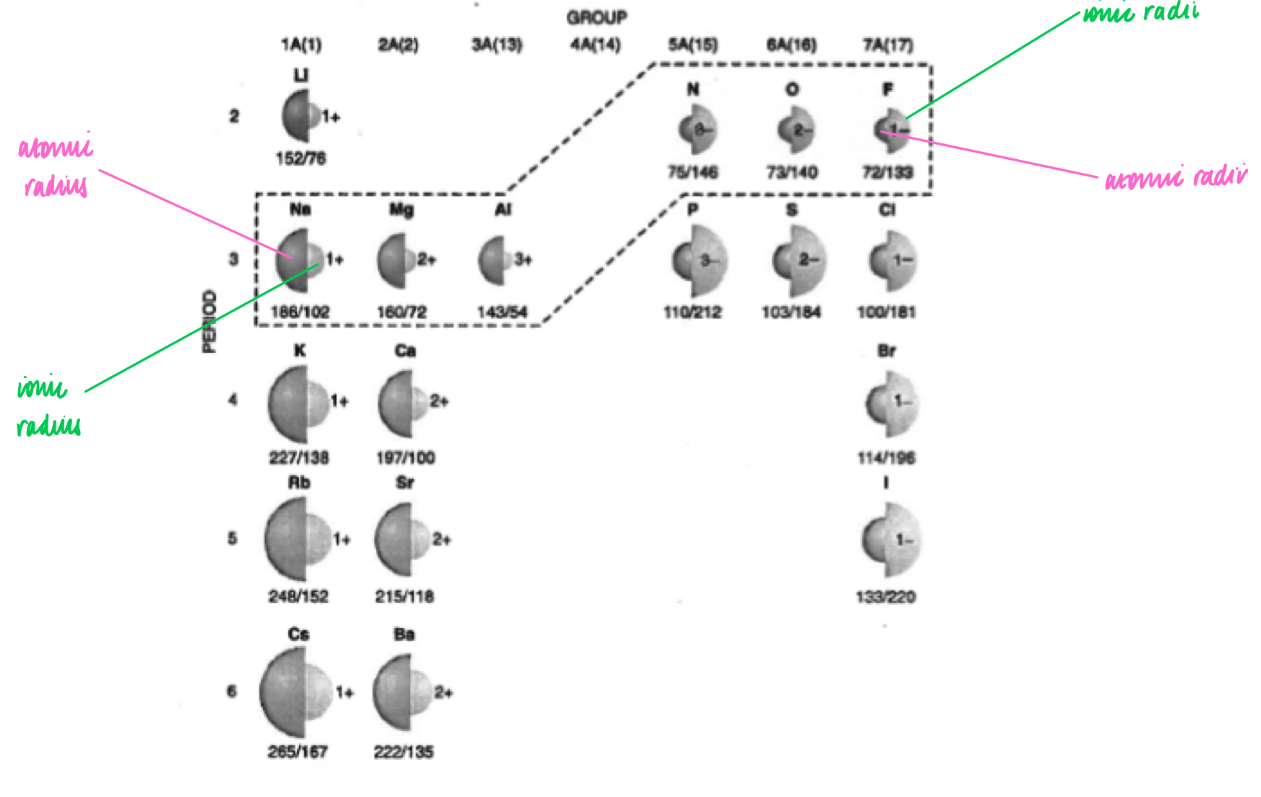
Reasons for the trends in ionic radii down a group and for a set of isoelectronic ions, e.g. N3- to Al3+
nuclear charge increases
The shielding remains the same
Therefore the attraction between the positive nucleus and outermost electrons increases
Therefore the distance between them increases
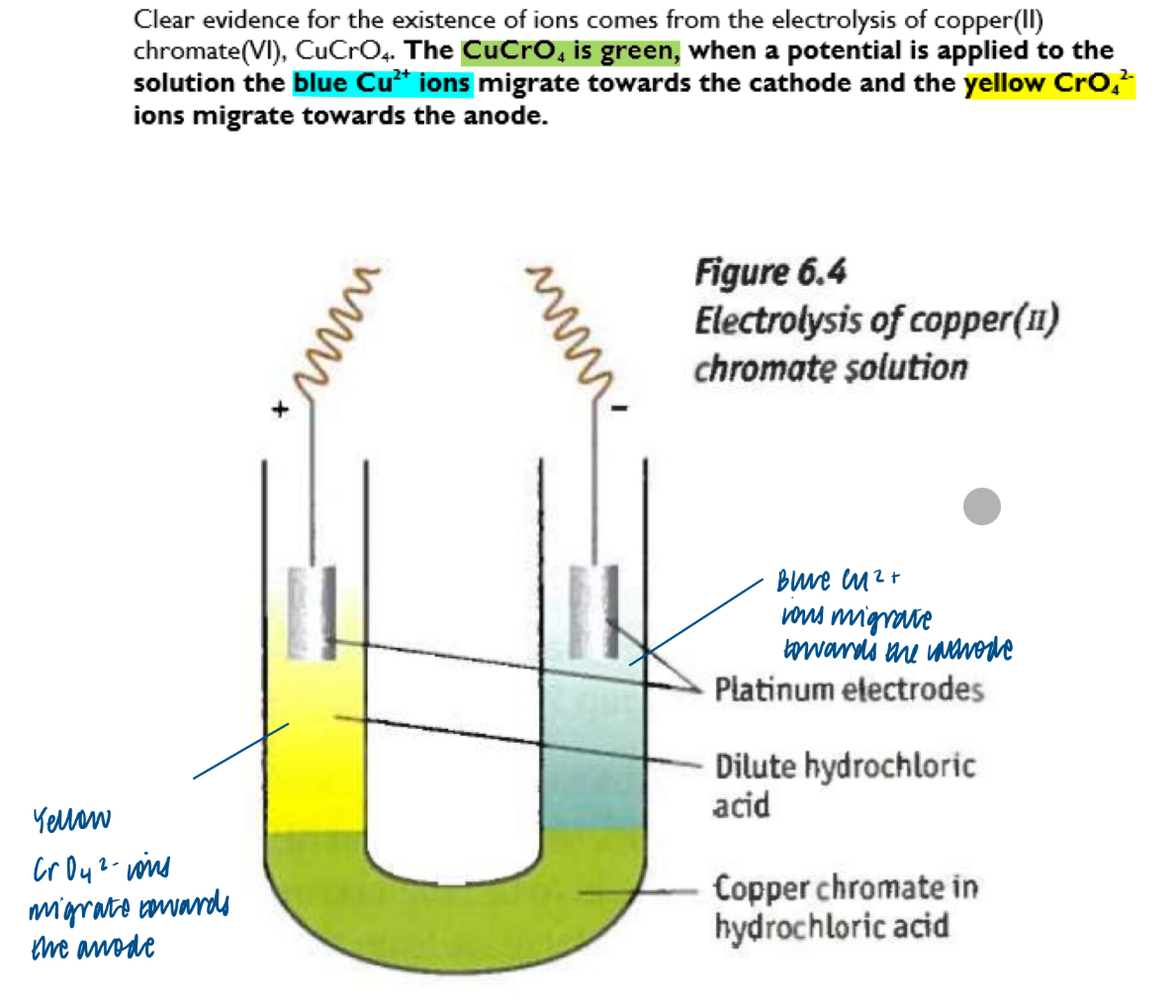
Physical properties of ionic compounds and the migration of ions in providing evidence for the existence of ions
Other evidence for the existence of ionic bonding comes from electron density maps. In ions, there is no shared electron density, as shown on electron density maps. However, in covalently bonded substances, shared electron density can be seen.
Covalent bonding
the strong electrostatic force of attraction between two nuclei and a shared pair of electrons between them
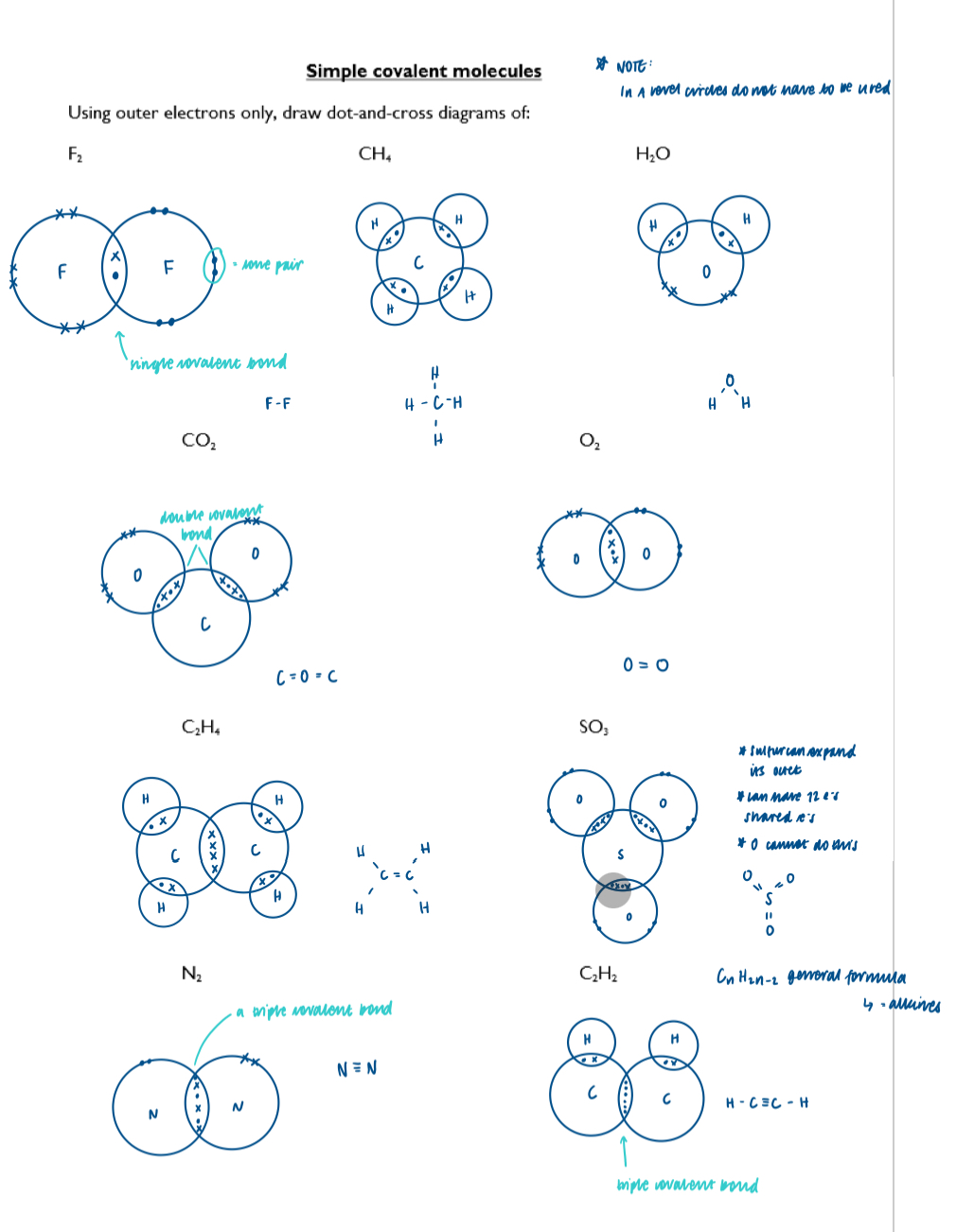
Draw dot-and-cross diagrams to show electrons in covalent substances, including molecules with single, double and triple bonds
Expanding the octet:
When forming covalent bonds, elements in period 3 onwards can have more than a share of 8 electrons in their outer shell.
E.g. sulfur:
16S [Ne] 3s2 3p4
This is known as the ground state of electronic configuration. There are 2 unpaired electrons so S can form 2 covalent bonds. Sometimes, it does only form 2 bonds, e.g. in H2S, but other times its expands its octet. The reason S can do this is because it has an empty 3D subshell, quite close in energy, so electrons can get excited into the 3D subshell.
Energy is neeeded to promote / excite the electrons into empty orbitals but this is more than compensated for the energy released when greater number of new bonds formed.
N.B. Elements in period 2 cannot expand their octets because there are no available empty orbitals I.e. 2D subshells do not exist. Too much energy would be needed to promote / excite electrons into orbitals in the next shell.
![<p>When forming covalent bonds, elements in period 3 onwards can have more than a share of 8 electrons in their outer shell. </p><p>E.g. sulfur:</p><p>16S [Ne] 3s2 3p4</p><p>This is known as the ground state of electronic configuration. There are 2 unpaired electrons so S can form 2 covalent bonds. Sometimes, it does only form 2 bonds, e.g. in H2S, but other times its expands its octet. The reason S can do this is because it has an empty 3D subshell, quite close in energy, so electrons can get excited into the 3D subshell.</p><p>Energy is neeeded to promote / excite the electrons into empty orbitals but this is more than compensated for the energy released when greater number of new bonds formed. </p><p></p><p>N.B. Elements in period 2 cannot expand their octets because there are no available empty orbitals I.e. 2D subshells do not exist. Too much energy would be needed to promote / excite electrons into orbitals in the next shell. </p>](https://knowt-user-attachments.s3.amazonaws.com/0cd0a9d2-d3ab-4668-9b52-1b912b74651d.jpg)
Draw dot-and-cross diagrams to show electrons in covalent substances, including Species exhibiting dative covalent bonding, including Al2Cl6 and ammonium ions
A dative covalent bond is formed when both electrons in the bond come from the same atom.

Relationship between bond lengths and bond strengths for covalent bonds
Shorter the bond length, the greater the bond strength and vice versa
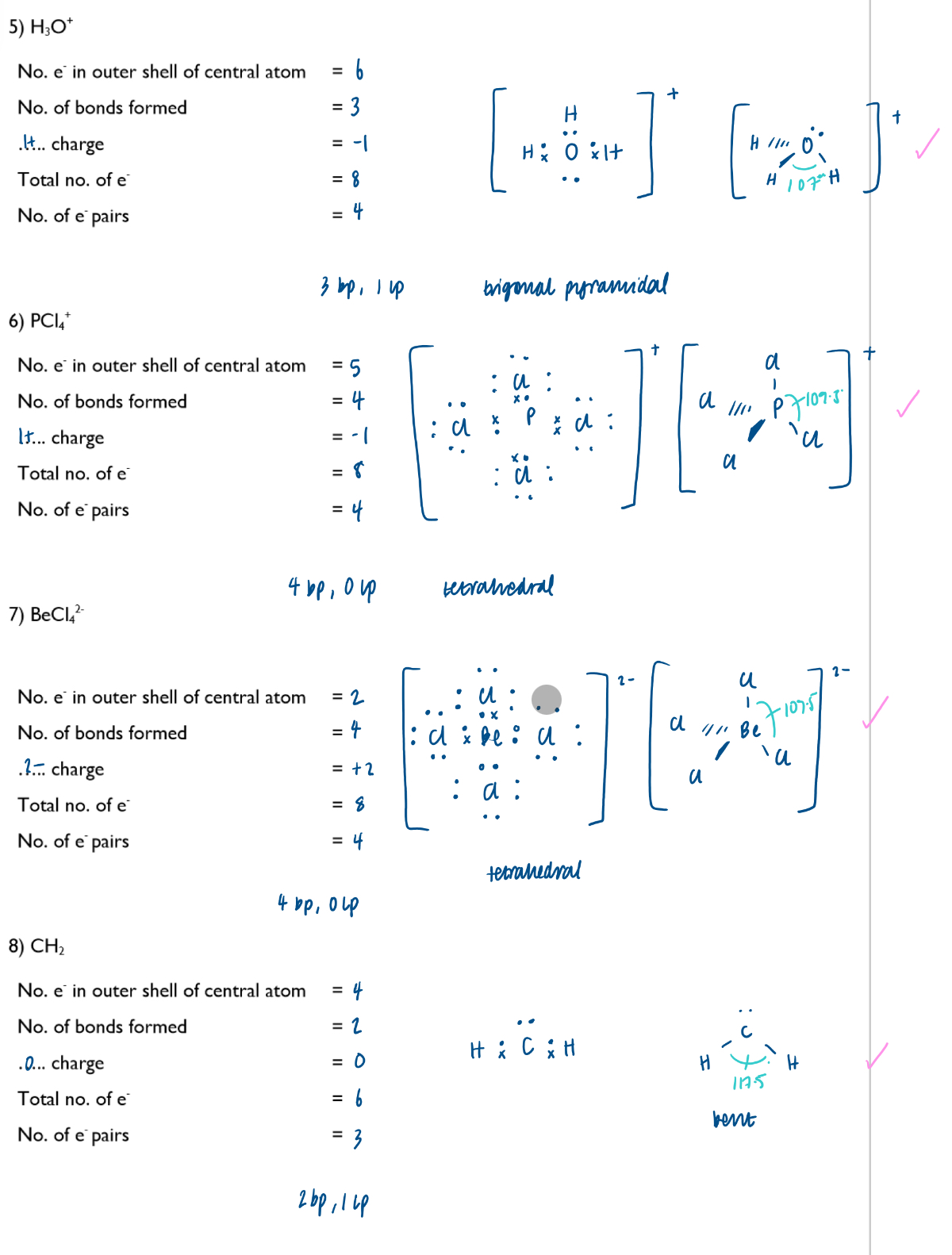
Shape of a simple molecule:
Electron pairs repel to positions of maximum separation and therefore minimum repulsion (the electron-pair repulsion theory)
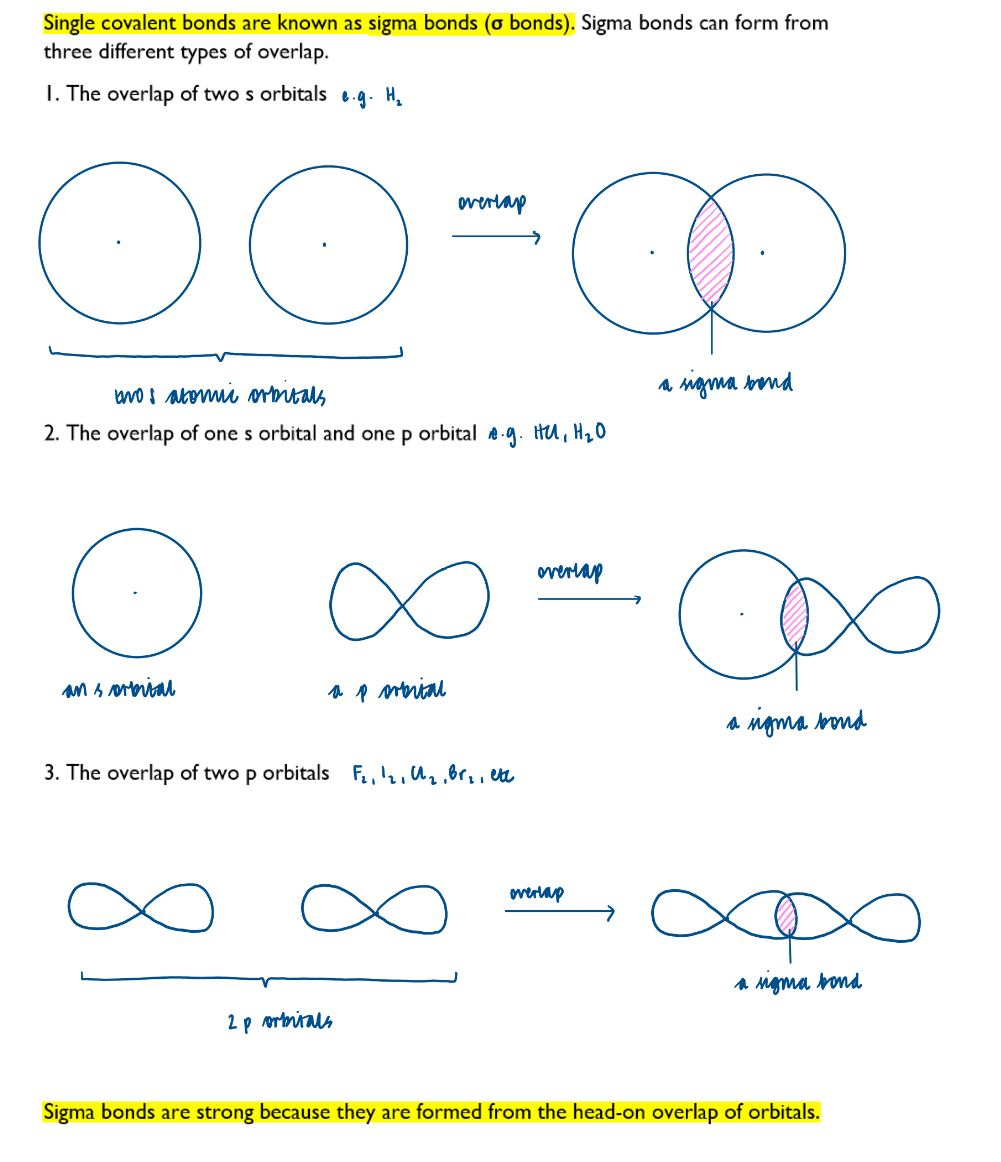
How single covalent bonds form:
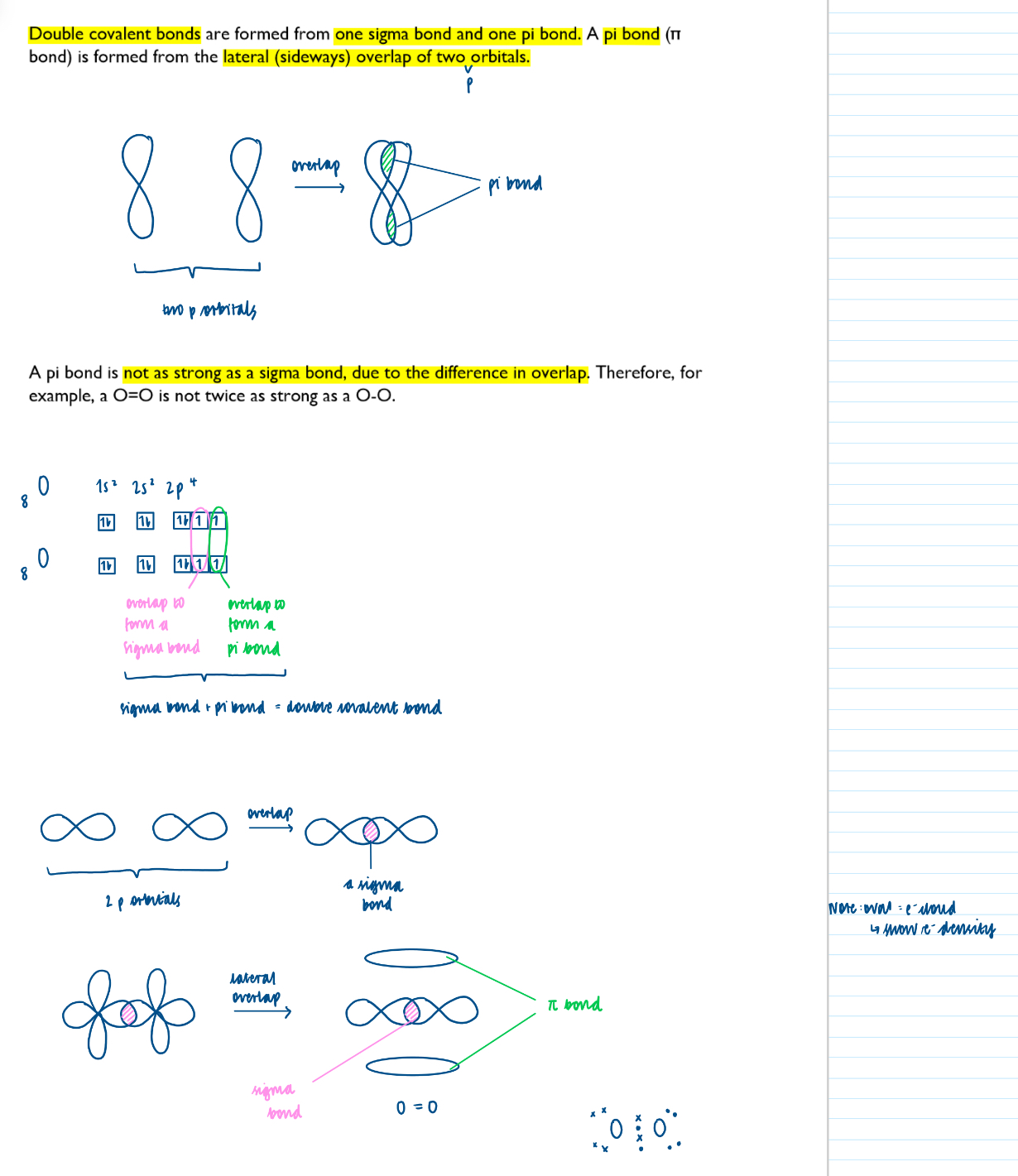
How double covalent bonds form:
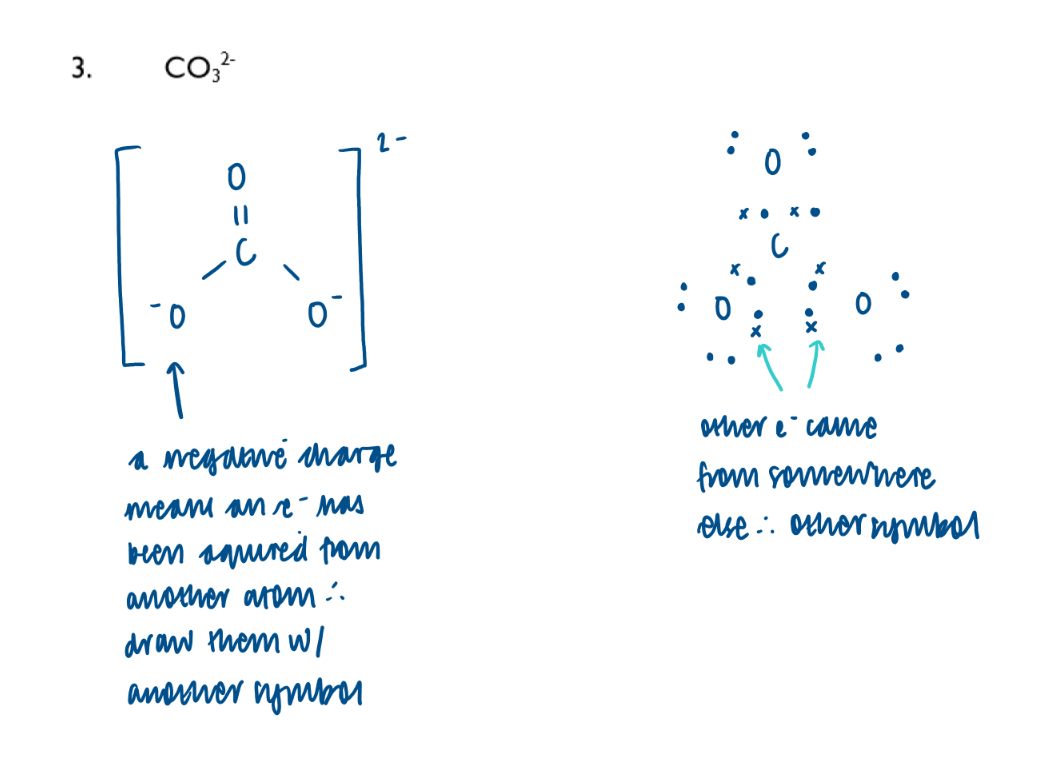
Carbonate ion dot-and-cross diagram:
Nitrate ion dot-and-cross diagram:
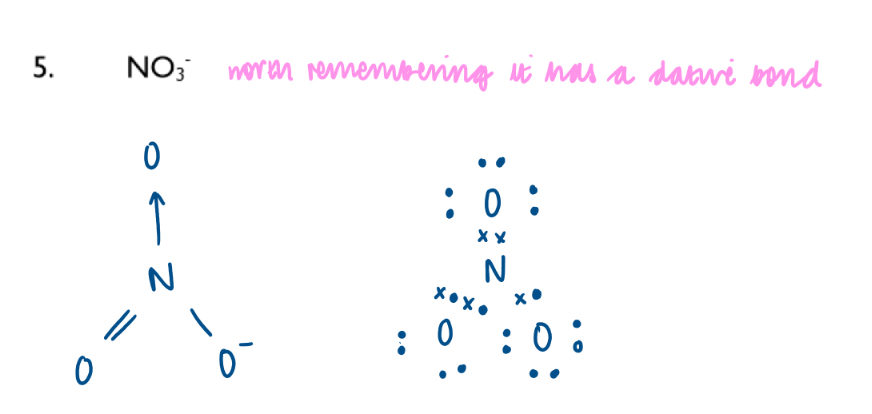
Sulphate ion dot-and-cross diagram:
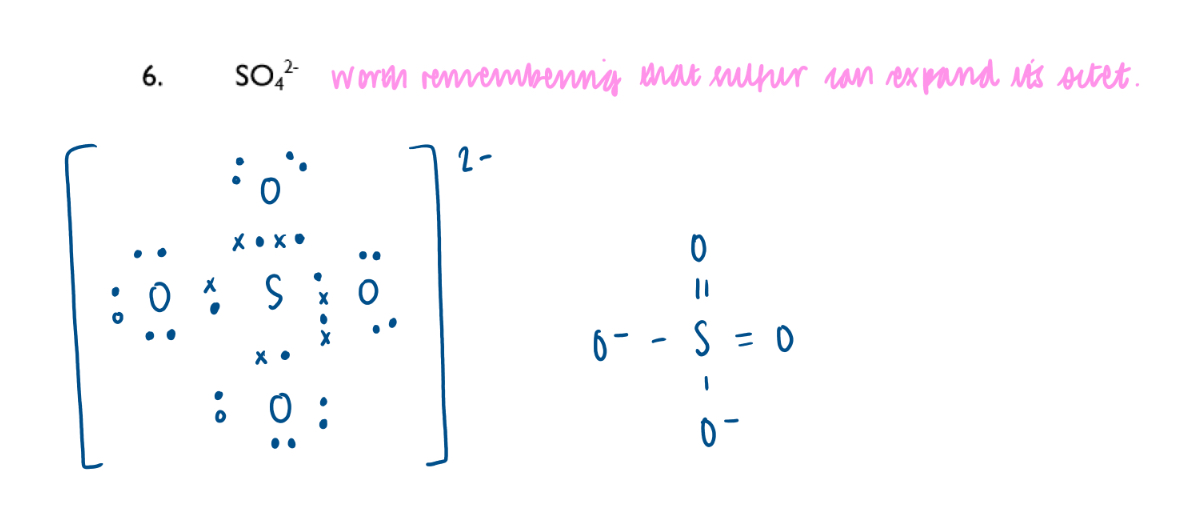
Predict the shapes of, and bond angles in, simple molecules and ions using the electron-pair repulsion theory
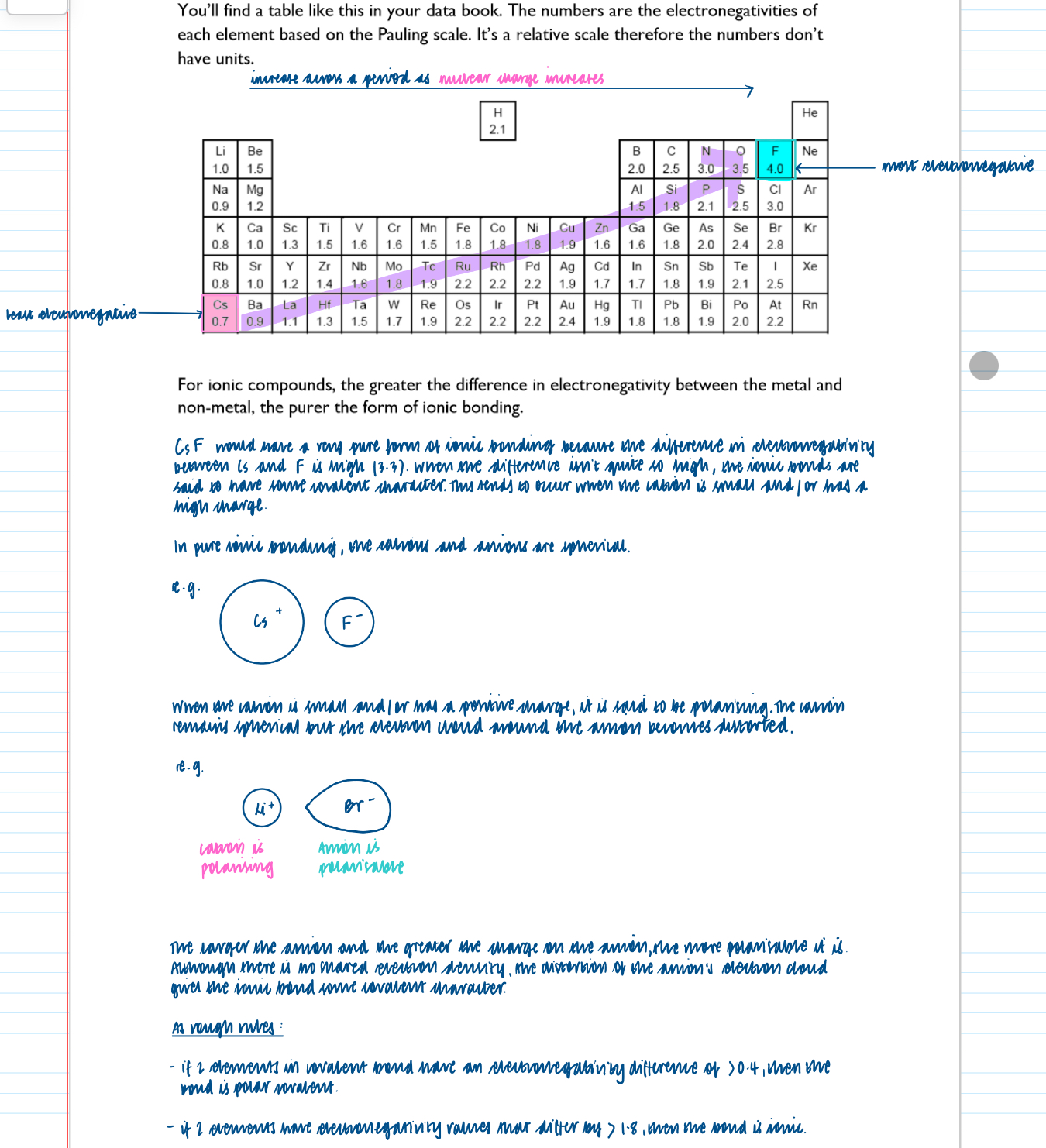
What is electronegativity?
The ability of an atom to attract the shared pair of electrons in a covalent bond
Ionic and covalent bonding are extremes of a continuum of bonding type and electronegativity differences lead to bond polarity in bonds and molecules
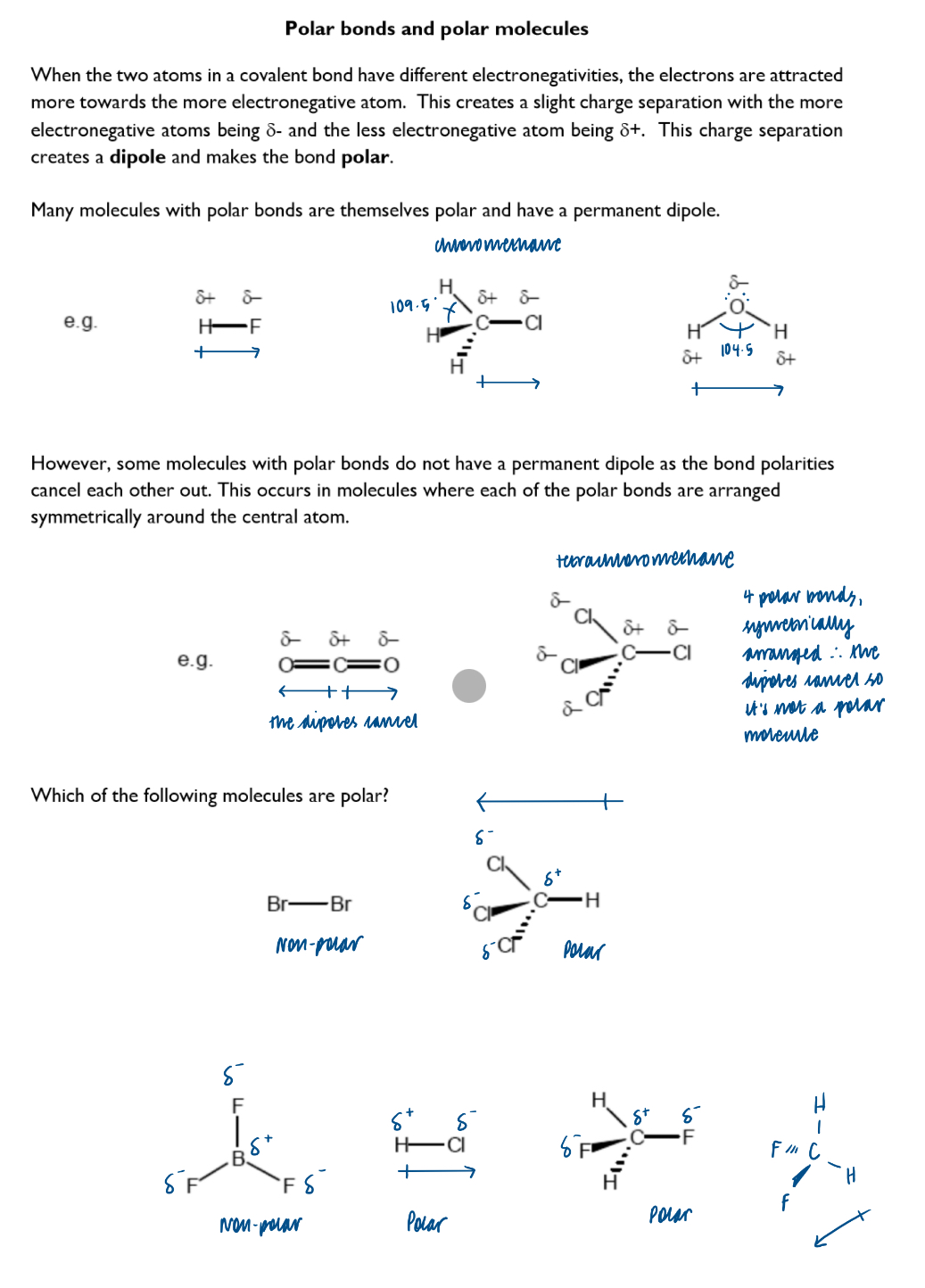
Molecules with polar bonds may not be polar molecules and be able to predict whether or not a given molecule is likely to be polar:
Nature of intermolecular forces resulting from the following interactions: London forces
uneven distribution of electrons / random movement of electrons / random fluctuations of electrons
Results in an instantaneous dipole / temporary dipole in the first molecule
Cause / induces a second dipole on another molecule
Nature of intermolecular forces resulting from the following interactions: Permanent dipoles
A polar molecule has a permanent dipole ( the separation of charge in a molecule).
All simple covalent molecules have London forces but molecules with permanent dipoles also have permanent dipole - permanent dipole (pd-pd) forces. The force of attraction between the positive end of one molecule and the negative end of another molecule. For example, in hydrogen chloride the delta plus H on one molecule is attracted to the Cl delta negative of another.

Nature of intermolecular forces resulting from the following interactions: Hydrogen bonds
A hydrogen bond is an especially strong kind of permanent dipole- permanent dipole force in which a hydrogen atom serves as a kind of bridge between two electronegative atoms.
A hydrogen bond is the electrostatic force of attraction between the poorly shielded proton of a hydrogen atom bonded to a small electronegative atom (either fluorine, oxygen, or nitrogen) and a lone pair of electrons on a neighbouring molecule.
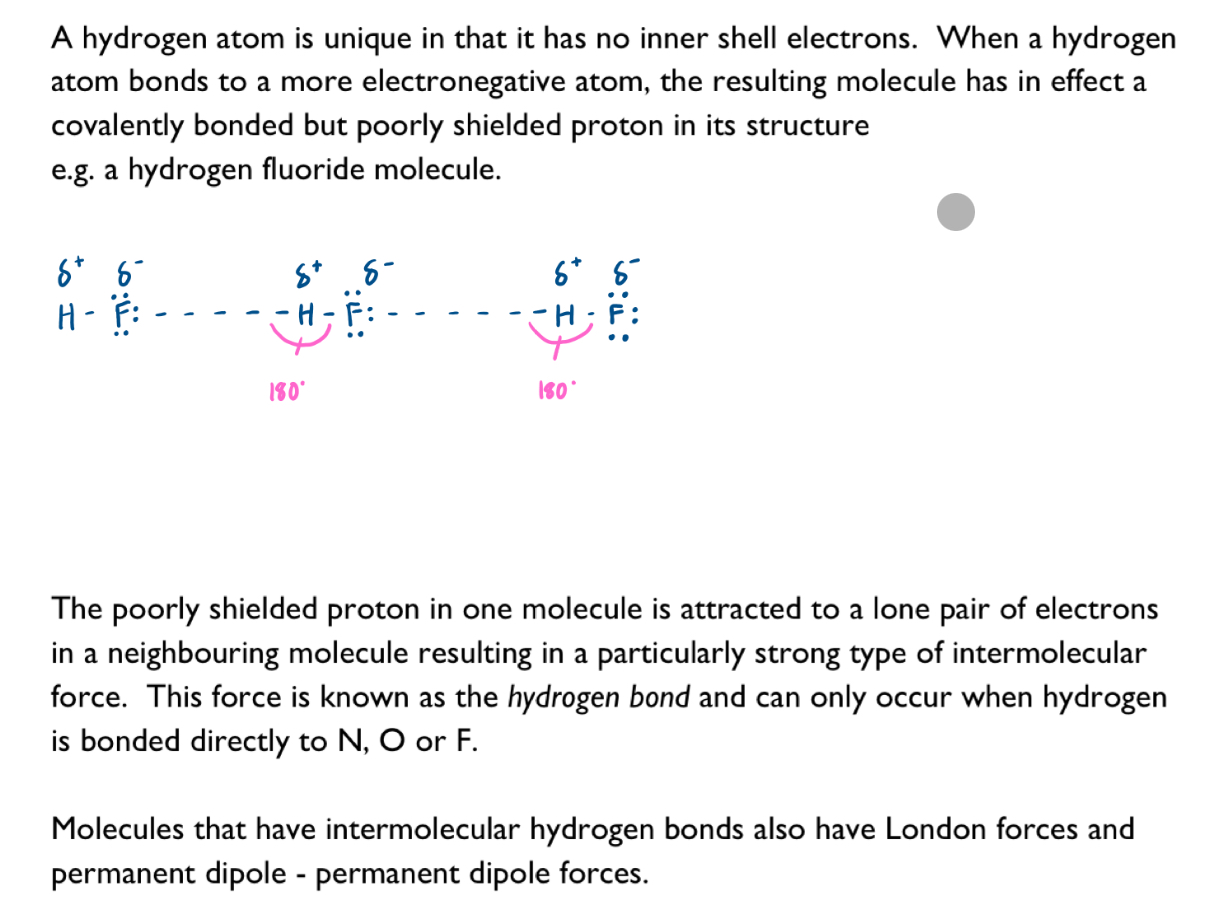
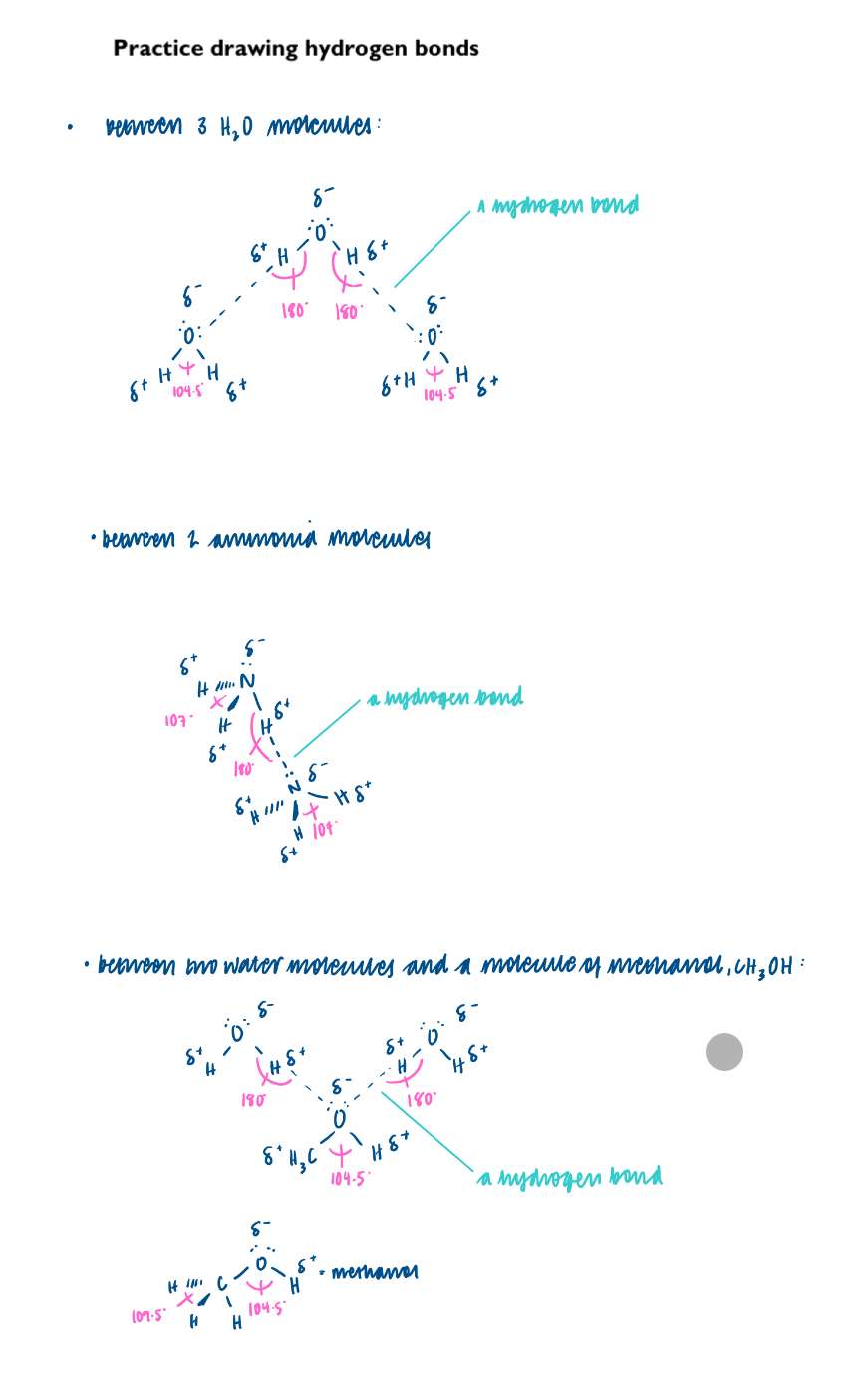
Interactions in molecules, such as H2O, liquid NH3 and HF, giving rise to hydrogen bonding
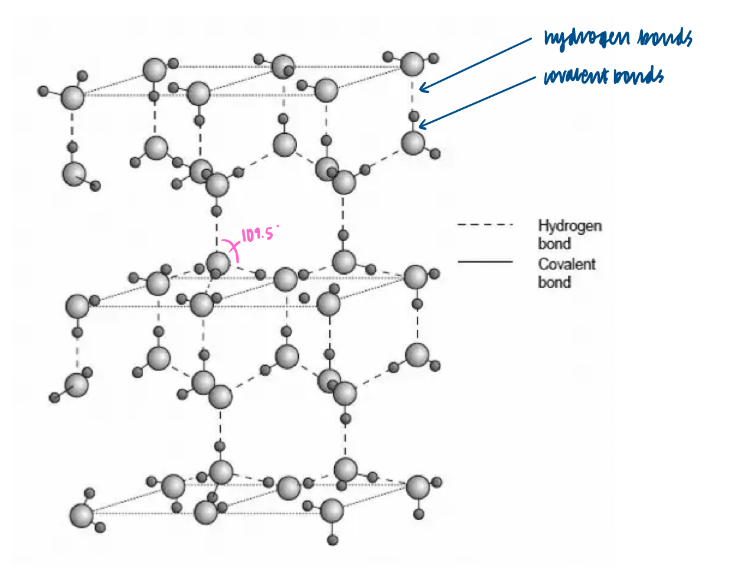
Why does water have a relatively high melting temperature and boiling temperature (because of hydrogen bonding)?
Due to hydrogen bonding, the melting and boiling temperatures of water are significantly higher than expected, as they require relatively much more energy than other intermolecular forces (like London forces) to be overcome.
Why is the density of ice lower than that of water (due to hydrogen bonding)?
Unlike other intermolecular forces, hydrogen bonding is directional. The shape of the three dimensional structure is governed by the position of lone pairs of electrons. The presence of two lone pairs and two hydrogen atoms results in a three dimensional tetrahedral structure in ice.
In ice the molecules are held further apart by the hydrogen bonds than in water. This explains the low density of ice compared to water.
The arrangement of the H2O molecules in ice creates a very open structure. In the ice lattice each molecule has 4 neighbours. When ice melts, the lattice breaks up and the water molecules can pack together more closely. In the liquid phase, some molecules have 4 near neighbours while others have 5 (average number is 4.4). This is why, when H2O freezes, it expands by 9% and why ice is less dense than water at 0 degrees Celsius.
Trend in boiling temperatures of alkanes with increasing chain length
As the chain length increases, the boiling temperature increases.
longer chains have larger surface areas, therefore more points of contact between molecules, therefore stronger London forces
These need more energy to be overcome
The effect of branching in the carbon chain on the boiling temperatures of alkanes
The more branching, the lower the boiling temperature.
less points of contact between molecules
Therefore weaker London forces
The relatively low volatility (higher boiling temperatures) of alcohols compared to alkanes with a similar number of electrons
hydrogen bonding in alcohols
Therefore more energy is needed to overcome the relatively much stronger hydrogen bonds compared to the weak London forces between molecules
So alcohols have a relatively low volatility / high boiling temperature compared to alkanes with similar number of electrons
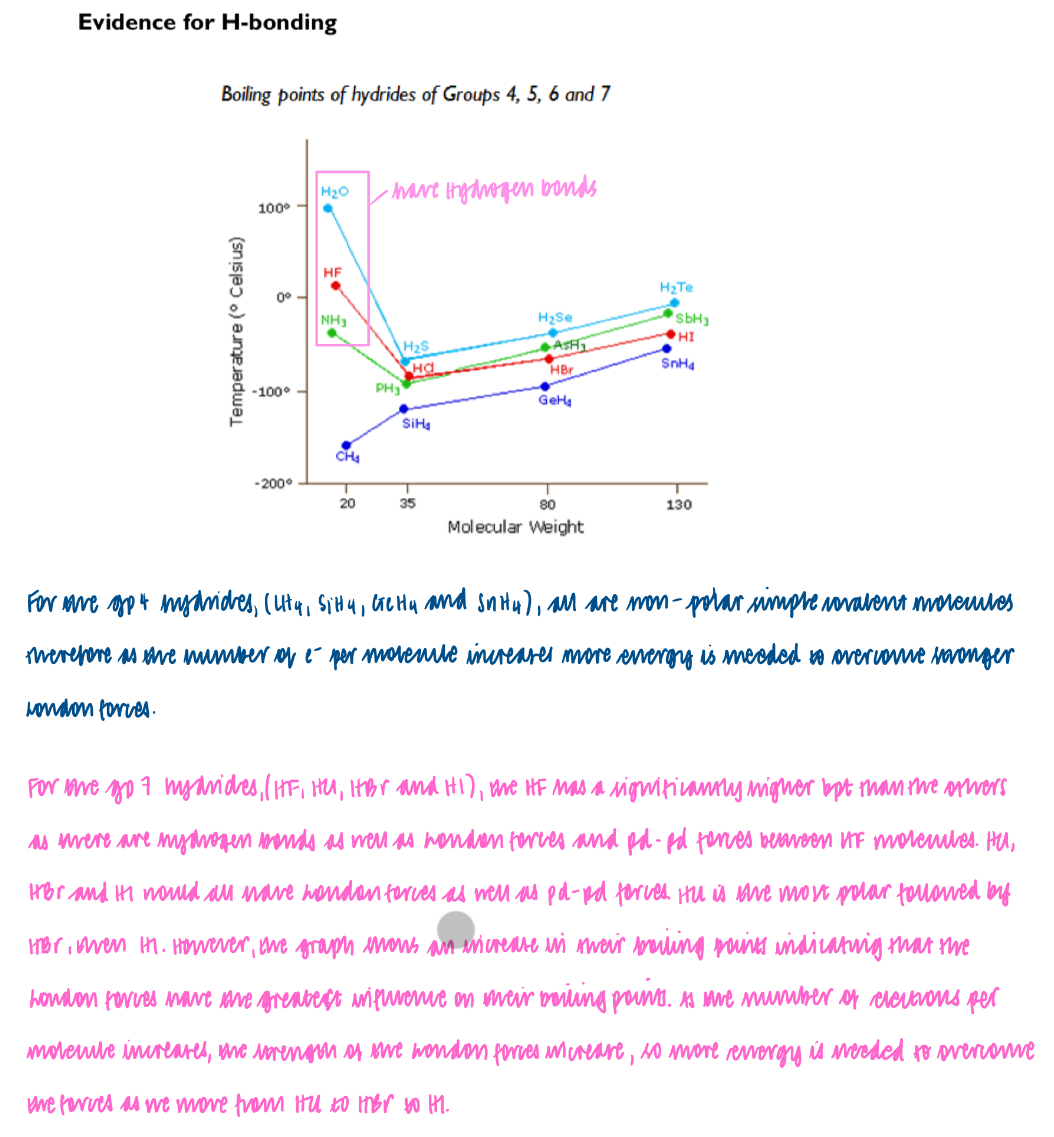
Trends in boiling temperatures of the hydrogen halides, HF to HI
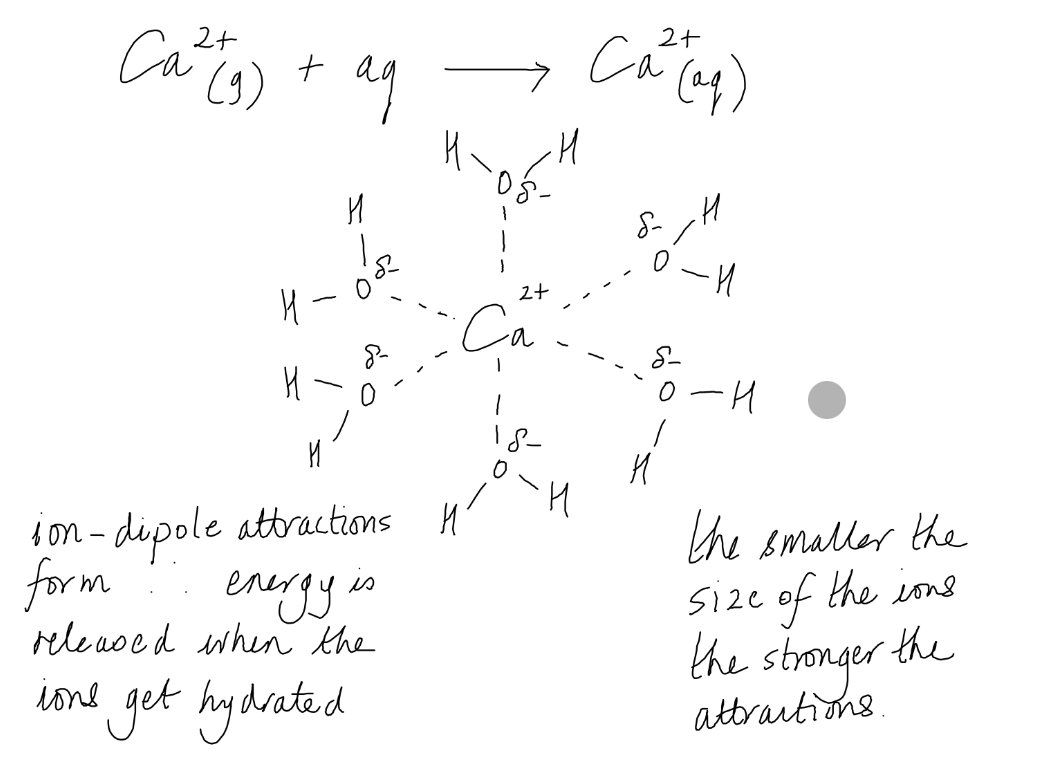
Water in dissolving ionic compounds, in terms of hydration of the ions
Many ionic compounds dissolve in water.
This is because more energy is released when the attractions form between the polar molecules and the ions than is needed to break the strong electrostatic forces of attraction between the ions in the lattice.
The ions become hydrated.
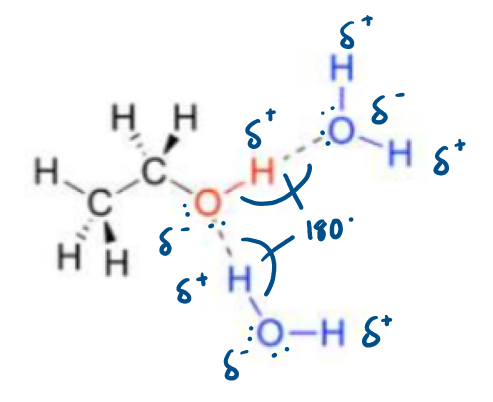
Water in dissolving simple alcohols, in terms of hydrogen bonding
Usually dissolve in water.
Usually dissolve well as hydrogen bonds will form between the solvent and solvent molecules.
As the carbon chain length increases, the solubility in water decreases.
The hydrogen bonds between the molecules are a similar strength to those that form between water molecules.
Water as a poor solvent for compounds (to include polar molecules such as halogenoalkanes), in terms of inability to form hydrogen bonds
Usually insoluble or slightly soluble.
It would be energetically unfavourable for the hydrogen bonds between water molecules to break, only to be replaced by weaker London forces.
Non-aqueous solvents, for compounds that have similar intermolecular forces to those in the solvent
Non-polar covalent molecules usually dissolve well as London forces will form between the solvent and solute molecules.
Metallic bonding
the strong electrostatic force of attraction between metal ions and delocalised electrons
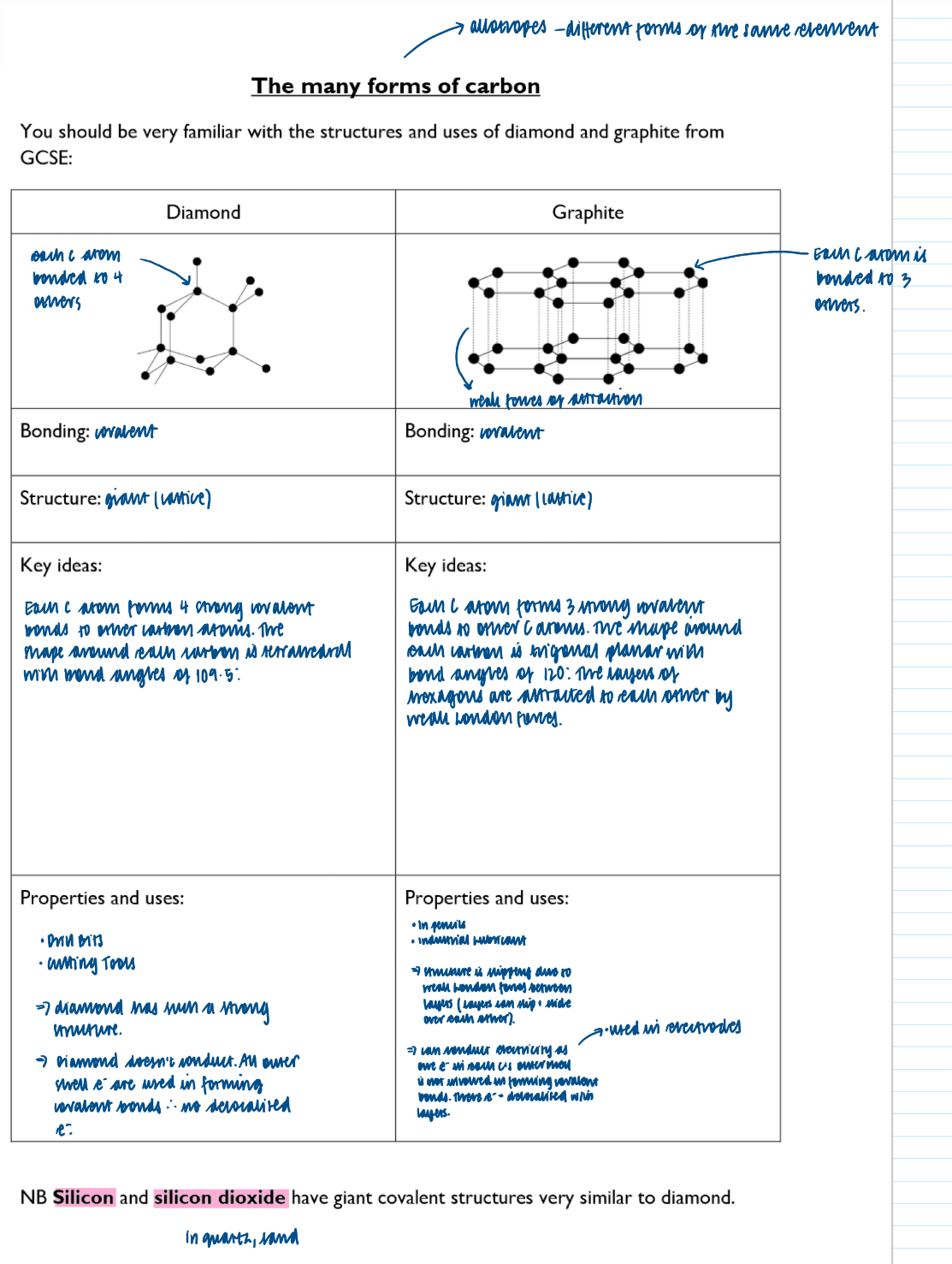
Where a giant lattices present?
ionic solids (giant ionic lattices)
Covalently bonding solids, such as diamond, graphite and silicon (IV) oxide (giant covalent lattices)
Solid metals (giant metallic lattices)
Structure of covalently bonding substances, such as iodine, I2, and ice H20, is?
Simple molecular
Different structures formed by carbon atoms, including graphite, diamond and graphene
Graphene:
graphene comprises of one layer of graphite, so each carbon atom is covalently bonded to 3 others and so the 4th outer electron belonging to each atom is delocalised
Graphene therefore can conduct electricity just like graphite
It’s a better conductor than copper
It is the thinnest material known to man but also one of the strongest
Fullerenes:
carbon also exists as simple covalent molecules in the form of fullerenes
Fullerenes are made from carbon atoms joined together to make cages or tubes
The cages are spherical molecules with formula C32, C50, C60, C70, C76, C84 etc
The most commonly known fullerenes is C60 which is named as ‘buckminsterfullerene’ after the designer of the geodesic dome
Each C atom is bonded to three others
As the structure has to bend to form a cage, the bond angles are <120 degrees
The 4th outer shell electron of each carbon is delocalised, thus fullerenes can conduct electricity to some extent. Each molecule can but the delocalised electron cannot nove to neighbouring molecules so the conductivity is limited.
Predict the type of structure and bonding present in a substance from numerical data and/or other information
if two elements have electronegativity values that differ by >1.8, then the bond is ionic
Predict the physical properties of a substance, including melting and boiling temperature, electrical conductivity and solubility of water, in terms of: the type of particle present (atoms, molecules, ions, electrons)
Predict the physical properties of a substance, including melting and boiling temperature, electrical conductivity and solubility of water, in terms of: The structure of the substance
Predict the physical properties of a substance, including melting and boiling temperature, electrical conductivity and solubility of water, in terms of: The type of bonding and the presence of intermolecular forces, where relevant