PHARM-Hematology Agents Flashcards- IRAT 7
1/165
Earn XP
Description and Tags
Pharmacology of Hematology Agents
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
166 Terms
What are the 6 Classes of Hematology Agents?
Iron Homeostasis & Therapy
Anemia-Correcting Agents
Sickle Cell Disease–Targeted Agents
Complement and Immune Modulators
Bone Marrow Stimulation & Androgenic Agents
Gene Therapy
Iron Homeostasis & Therapy:
What are the Oral Iron Supplements? (*5)
Ferrous Sulfate
Ferrous gluconate
Ferrous Fumarate
Iron Protein succinylate
Ferrous bisglycinate
What is the elemental iron content of ferrous sulfate?
20–30% elemental iron per mg ferrous sulfate salt (varies by manufacturer).
What is the efficacy/cost profile of ferrous sulfate?
Least expensive and most common oral iron supplement.
What are the adverse effects of ferrous sulfate?
Taking with food increases tolerability but decreases absorption by 40–66%.
What is the elemental iron content of ferrous gluconate?
10–14% elemental iron per mg ferrous gluconate salt.
What is the efficacy/cost of ferrous gluconate?
More expensive than ferrous sulfate.
What are the adverse effects of ferrous gluconate?
Often sold in liquid form; better absorbed than ferrous sulfate tablets. Higher dosage may be needed due to less elemental iron.
What is the elemental iron content of ferrous fumarate?
33% elemental iron per mg ferrous fumarate salt.
What is the efficacy/cost of ferrous fumarate?
Similar efficacy to ferrous sulfate and ferrous gluconate.
What are the adverse effects of ferrous fumarate?
Similar GI adverse effects to ferrous sulfate and ferrous gluconate.
What is the composition of iron protein succinylate?
Ferric iron bound to protein; separates into elemental iron in intestines instead of stomach.
What is the efficacy/cost of iron protein succinylate?
More expensive than iron salts.
What are the adverse effects of iron protein succinylate?
May cause fewer GI effects (e.g., metallic taste); lowest adverse-effect rate; efficacy comparable to iron salts.
What is the cost and effect profile of ferrous bisglycinate?
More expensive than iron salts; may cause fewer GI adverse effects.
What are the 4 Oral Iron Supplements we reviewed?
Ferrous sulfate
Ferrous fumarate
Ferrous gluconate
Extended-release ferrous sulfate with mucoprotease
What are the main indications for oral iron supplements?
Iron deficiency anemia due to chronic blood loss, malnutrition, or pregnancy
Postpartum anemia
Menstruation-related anemia
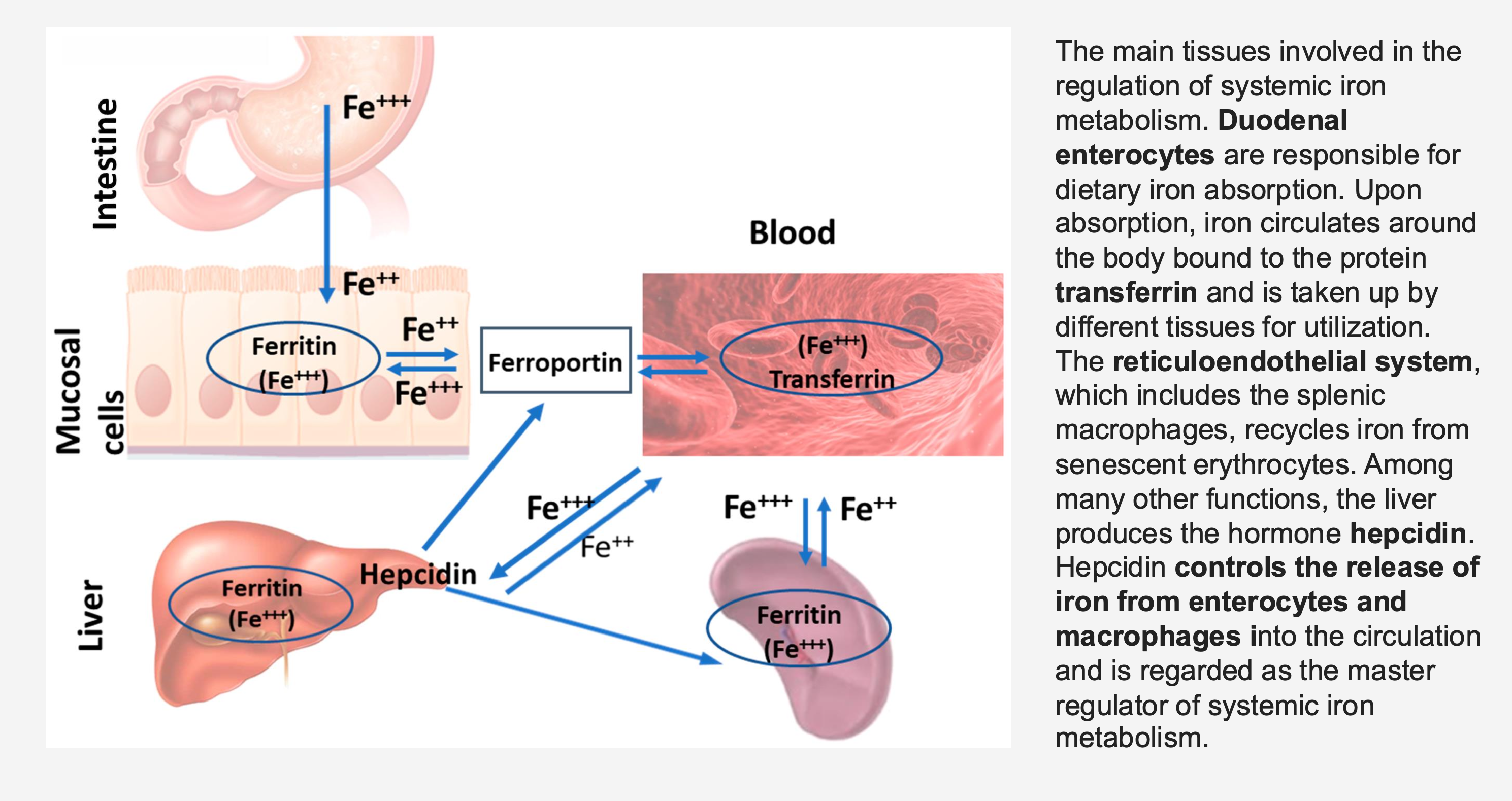
Which cells are responsible for dietary iron absorption?
Duodenal enterocytes.
What protein binds absorbed iron for transport in the blood?
Transferrin.
What system recycles iron from old red blood cells?
The reticuloendothelial system (includes splenic macrophages).
What organ produces the hormone hepcidin?
The liver.
What is the function of hepcidin?
Controls the release of iron from enterocytes and macrophages into circulation; master regulator of systemic iron metabolism.
What protein exports iron from mucosal cells to the blood?
Ferroportin.
What protein stores iron within cells?
Ferritin.
What is the MOA of Oral Iron Supplements? (Ferrous sulfate, Ferrous fumarate, Ferrous gluconate, Extended-release ferrous sulfate with mucoprotease)
Provides elemental iron → absorbed in duodenum → incorporated into hemoglobin and myoglobin.
What is the main purpose of oral iron supplements?
Restores oxygen-carrying capacity.
How do oral iron supplements affect RBC production?
Stimulate erythropoiesis (MB making new RBCs)
Why is every-other-day dosing recommended?
🧠 It optimizes absorption by reducing the rise in hepcidin, preventing iron from getting trapped in ferritin, so more iron enters the blood.
💪 It also helps reduce constipation, a common side effect of iron therapy.
What are common adverse effects of oral iron supplements?
Nausea, epigastric discomfort
Constipation, dark stools (could not be an active bleed)
Reduced absorption when taken with food, calcium, or PPIs — advise patients to take iron on an empty stomach for best results.
🚫 Avoid taking with calcium, dairy, or antacids (PPIs).
🍊 Vitamin C or citrus juice enhances absorption.
What are contraindications for oral iron supplements?
🩸 Hemochromatosis – Body absorbs too much iron → iron builds up in organs.
🧲 Hemosiderosis – Extra iron from transfusions or supplements → iron stored in tissues.
🧬 Hemolytic Anemia – Red blood cells break down too early → releases excess iron.
**don’t want to give iron to pts who have too much iron OR iron overload
🫀 Chronic Liver Disease with Iron Overload – Damaged liver can’t handle iron → iron accumulates and worsens liver damage.
What are the Parenteral Iron Preparations (IV or IM)?
Iron sucrose
Ferric carboxymaltose
Ferumoxytol
Low-molecular-weight iron dextran
What is the MOA of parenteral iron?
⚙ Provides IV elemental iron complexed with a carbohydrate shell → bypasses GI absorption.
What is the main purpose of parenteral iron therapy?
💪 Rapidly replenishes iron stores.
When is parenteral iron used?
🩸 Used when oral iron is not tolerated or ineffective.
What are the common adverse effects of parenteral iron?
1. Hypotension
2. Arthralgia
3. Infusion reactions
4. Nausea, back pain, headache
What are the contraindications for parenteral iron therapy?
Iron overload disorders🚫
Active infection
Hypersensitivity
🩸 Comparison of Iron Therapies
💊 Type | ⚙ Main Purpose | 💡 When Used | 💉 Route | ⚠ Key Points / Differences |
|---|---|---|---|---|
Oral Iron Supplements (Ferrous sulfate, fumarate, gluconate) | 🩷 Adds iron to treat deficiency | Iron deficiency anemia (chronic blood loss, pregnancy, poor diet) | Oral (PO) | ✅ First-line for most patients🚫 Absorption ↓ with food, Ca, PPIs🍊 Better absorbed with vitamin C💩 Constipation & dark stools common |
Parenteral Iron (Iron sucrose, ferric carboxymaltose, ferumoxytol) | 💪 Adds iron directly into bloodstream | When oral iron is not tolerated, ineffective, or malabsorbed | IV | ⚡ Rapid iron repletion❌ Risk: hypotension, infusion reactionsUsed for severe deficiency or CKD |
Iron Chelation Therapy(Deferoxamine, Deferasirox, Deferiprone) | 🧲 Removes excess iron | Iron overload (e.g., thalassemia, chronic transfusions) | IV, SQ, or PO (depends on drug) | 🧪 Binds excess iron → excreted in urine/feces🚫 Used only when Fe stores are too high⚠ Risk: GI upset, hepatic/renal toxicity |
🧲 What are the 3 types of Iron Chelation Therapy (used to lower iron levels)?
1⃣ Deferoxamine (IV/SQ) – Binds iron 1:1; excreted mostly in urine; not orally available.
2⃣ Deferasirox (PO) – Binds iron 2:1; excreted in feces; taken once daily.
3⃣ Deferiprone (PO) – Binds iron 3:1; excreted in urine; best for removing intracellular and cardiac iron.
What are the indications for Iron Chelation Therapy?
Chronic transfusion-related iron overload (thalassemia, sickle cell, myelodysplasia).
Iron overload syndromes.
What is the main MOA of Iron Chelation Therapy?
Chelates free iron (Fe³⁺) → forms stable complexes excreted in urine or feces → prevents oxidative tissue injury and organ damage from iron overload.
Questions: What is the mechanism of action of Deferoxamine (IV/SQ)?
What is the drug-to-iron binding ratio for Deferoxamine?
How is Deferoxamine excreted?
From which iron stores does Deferoxamine chelate iron?
What is the route of administration for Deferoxamine?
What unique action does Deferoxamine have on ferritin?
Answers
1:1 (one drug molecule binds one iron atom).
Mostly in urine, with some excretion in feces.
From ferritin and hemosiderin (not from transferrin or hemoglobin).
Parenteral only (IV or SubQ) — not orally bioavailable.
Induces ferritin degradation via lysosomal autophagy.
What is the MOA of Deferasirox (Oral)?
Questions
What is the drug-to-iron binding ratio for Deferasirox?
How is Deferasirox excreted?
Is Deferasirox orally active, and what type of iron does it target?
What is the dosing frequency of Deferasirox, and why?
Answers
2:1 (two drug molecules bind one iron atom).
Excreted in feces.
Yes, it is orally active and selectively binds Fe³⁺ (ferric iron).
Once-daily dosing due to its long half-life.
What is the MOA of Deferiprone (Oral)?
Questions
What is the drug-to-iron binding ratio for Deferiprone?
How is Deferiprone excreted?
Is Deferiprone orally active, and does it enter cells?
What type of iron is Deferiprone especially effective at removing.
Answers
3:1 (three drug molecules bind one iron atom).
Excreted in urine.
Yes, it is orally active and can penetrate cells.
Effective for intracellular iron, especially cardiac iron.
What are the general adverse effects of iron chelation therapy?
GI distress and hepatic dysfunction.
Which adverse effects are specifically associated with Deferiprone?
Neutropenia, agranulocytosis, arthralgia, and zinc deficiency (especially in diabetic patients).
Which adverse effects are specifically associated with Deferoxamine?
Ototoxicity and visual changes.
Which adverse effects are associated with Deferoxamine and Deferasirox?
Renal impairment.
What are the main contraindications for iron chelation therapy?
Severe renal or hepatic disease, and hypersensitivity to the chelating agent.
A 28-year-old woman with heavy menstrual bleeding presents with fatigue and microcytic anemia. She is started on ferrous sulfate but complains of constipation and dark stools.
Question: What dosing strategy could optimize her iron absorption while minimizing gastrointestinal side effects?
Taking iron every other day helps reduce the rise in hepcidin, allowing for better absorption of iron and fewer GI side effects like constipation and dark stools.
62-year-old man with Crohn’s disease and prior small bowel resection continues to have iron-deficiency anemia despite oral supplementation. Question: Which iron preparation is most appropriate, and what is its main advantage compared to oral iron?
Answer:
➡ Parenteral (IV) iron — e.g., iron sucrose, ferric carboxymaltose, or ferumoxytol.
Explanation:
Because of malabsorption from Crohn’s disease and bowel resection, oral iron is ineffective. IV iron bypasses the GI tract, allowing for rapid and complete repletion of iron stores even when absorption is impaired.
A 19-year-old man with transfusion-dependent β-thalassemia shows rising ferritin levels and MRI evidence of cardiac iron overload.
Question: Which iron chelator is preferred for intracellular and cardiac iron clearance, and how is it administered?
Deferiprone (oral).
Preferred for intracellular and cardiac iron removal.
Penetrates cells effectively → iron excreted in urine.
What are the 3 Anemia-Correcting Agents?
Erythropoiesis-Stimulating Agents (ESAs)— Epoetin alfa, darbepoetin alfa
Folic Acid (B9)
Vitamin B12 (Cobalamin)—IM/SC cyanocobalamin or oral methylcobalamin
What are the main indications for erythropoiesis-stimulating agents (ESA)?- {Used For anemia of chronic disease}
Anemia of chronic disease (Chronic Kidney Disease (CKD), RA, IBD, HIV)
Chemotherapy-induced anemia ( *causes Cytopenia (a decrease in blood cell counts.) —> reduction in cell lines so agents that act like erythropoietin are used to stimulate the bone marrow to make more RBC)
Chronic kidney disease (CKD) with low eGFR
What is the mechanism of action of ESAs?
Mechanism of Action:
Recombinant erythropoietin analogs bind to erythropoietin receptors on erythroid progenitors → stimulate RBC production.
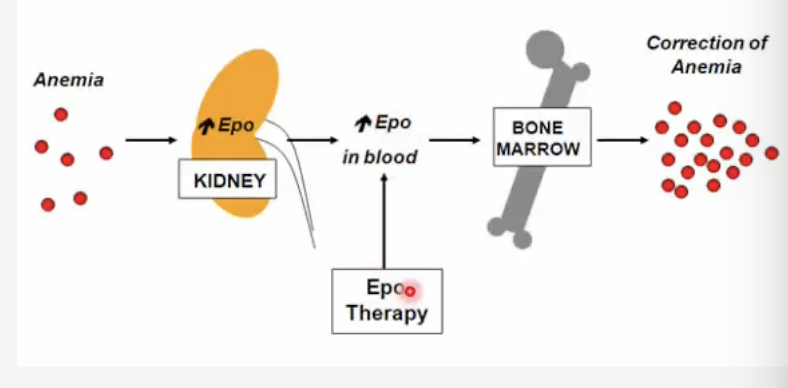
What is required for ESAs to be effective?
Adequate iron stores are needed.
What are common adverse effects of ESAs?
Hypertension
Thrombosis
Headache
Arthralgia
Risk of stroke if Hb > 12 g/dL [It means that if hemoglobin (Hb) levels rise above 12 g/dL while using erythropoiesis-stimulating agents (ESAs) like epoetin alfa or darbepoetin, there is a higher risk of blood clots and stroke 🧠⚠]
What are the contraindications for ESA use?
Uncontrolled hypertension
Pure red cell aplasia after ESA use
Quick Overview: Erythropoiesis-Stimulating Agents (ESAs)
Drugs: ?
Indications: ?
MOA: ?
Adverse: ?
Contraindications: ?
Drugs: Epoetin alfa, darbepoetin alfa
Indications: CKD anemia, chemo-induced anemia
MOA: Recombinant EPO binds receptors → RBC production (requires iron)
Adverse: HTN, thrombosis, stroke risk if Hb > 12 g/dL
Contraindications: Uncontrolled HTN, pure red cell aplasia
Epoetin alfa & darbepoetin alfa are what type of drugs?
Erythropoiesis-Stimulating Agents (ESAs)
🍃 Folic Acid (Vitamin B9) Flashcards
What is the typical daily dose of folic acid (B9)
1 mg PO daily
What is the MOA of folic acid?
Mechanism of Action:
Replaces folate → acts as a cofactor for thymidylate and purine synthesis → supports DNA synthesis and erythropoiesis.
What key biological processes does folic acid support?
Supports:
DNA synthesis 🧬
RBC formation ❤
Cell division and repair
What reactions is folic acid a cofactor for?
Cofactor for:
Thymidylate synthase (pyrimidine synthesis)
Purine biosynthesis
Homocysteine → methionine conversion (via MTHFR)
How is dietary folate and folic acid metabolized in the body (as shown in the diagram)?
Metabolism Pathway (from the diagram):
Folic acid (supplement) is converted by DHFR → DHF → THF (tetrahydrofolate)
THF is converted to 5,10-methylene-THF and 10-formyl-THF, which are used for DNA base (purine & thymidine) synthesis
Food folates enter as 5-MTHF, which participates in the homocysteine → methionine cycle for methylation reactions (SAM)
Folinic acid can bypass DHFR, entering directly as active THF derivatives
🧠 Summary Tip:
Folic acid → converted to THF → donates carbon units → makes DNA bases → supports RBC production and prevents megaloblastic anemia.
When would you give a patient Folic Acid?
Indications:
Megaloblastic anemia due to folate deficiency
Pregnancy, malnutrition, chronic alcoholism
To counteract methotrexate toxicity
How does methotrexate relate to folic acid metabolism?
Methotrexate relation:
Methotrexate inhibits dihydrofolate reductase (DHFR) → blocks THF formation → ↓ DNA synthesis.
Giving folic acid or folinic acid restores THF and prevents toxicity.
What are the possible adverse effects of folic acid?
Adverse Effects:
Rare hypersensitivity reactions
May worsen neurologic symptoms if vitamin B12 deficiency is present
What are the contraindications for folic acid use?
Contraindications:
Uncorrected vitamin B12 deficiency
Unknown cause of macrocytosis (should confirm before giving folate)
❌ Uncorrected Vitamin B12 Deficiency:
If you give folic acid when someone actually has a B12 deficiency, it can fix the anemia but not the nerve damage.
➡ This can mask the true problem and allow neurologic damage to worsen (like numbness, tingling, balance issues).
❌ Unknown Cause of Macrocytosis:
If you see large red blood cells (macrocytosis), you should find the cause first — it could be B12 deficiency, folate deficiency, or something else.
➡ Don’t give folate blindly, because it may hide B12 deficiency and delay proper treatment.
Anemia-Correcting Agents— Vitamin B12 (Cobalamin)
What are the different types and dosages of Vitamin B12 replacement
Types & Dosage:
Cyanocobalamin: 1000 mcg IM or SC weekly → then monthly maintenance
Methylcobalamin: 1 mg oral or sublingual daily
💡 Both forms restore B12 levels; parenteral used for absorption issues, oral for maintenance or mild deficiency.
What are the main indications for Vitamin B12 therapy?
Indications:
Pernicious anemia (autoimmune loss of intrinsic factor)
Post-gastrectomy or ileal resection
Vegan diet or malabsorption syndromes
What is the MOA of Vitamin B12?
Acts as a cofactor for DNA synthesis and myelin formation (allows for proper nerve function and conduction of electrical impulses)
Converts homocysteine → methionine (AA)—> BM to restore normal erythropoiesis (RBC production)
What are the adverse effects of Vitamin B12 supplementation?
Adverse Effects:
Hypokalemia during reticulocytosis (as RBCs regenerate)— *check K+ levels
Rash, acneiform eruptions, urticaria (rare)
What are the contraindications for Vitamin B12 therapy?
Contraindications:
Hypersensitivity to cobalt or cobalamin
Leber optic neuropathy (can worsen vision loss)
A 55-year-old woman with stage 4 CKD presents with symptomatic anemia (Hb 8.5 g/dL). She has adequate iron stores.
Question: Which pharmacologic agent should be initiated, and what is a major risk if her hemoglobin rises above 12 g/dL?
Answer:
➡ Erythropoiesis-Stimulating Agent (ESA) — such as Epoetin alfa or Darbepoetin alfa.
Explanation:
In CKD, decreased erythropoietin production leads to anemia.
ESAs stimulate the bone marrow to increase RBC production when iron stores are adequate.
Major Risk if Hb > 12 g/dL:
⚠ Increased risk of thrombosis and stroke due to blood becoming too viscous from excessive RBC production.
A 44-year-old vegan presents with megaloblastic anemia and neurologic symptoms (paresthesias, impaired vibration sense). Question: Which vitamin replacement is indicated, and why must folic acid alone not be used in this case?
Answer:
➡ Vitamin B12 (Cobalamin) replacement is indicated. Folate alone would correct anemia but worsen neurologic symptoms in B12 deficiency. B12 isessential for myelin and homocysteine → methionine metabolism.
Explanation:
The vegan diet lacks natural sources of Vitamin B12, leading to megaloblastic anemia and neurologic symptoms(due to myelin damage).
Giving folic acid alone may correct the anemia but will not fix the nerve damage — it can actually worsen neurologic symptoms by masking the true B12 deficiency.
Sickle Cell Disease– Targeted Agents
Hydroxyurea
L-Glutamine
Crizanlizumab (P-selectin inhibitor)
Voxelotor (HbS polymerization inhibitor)
What is the first line agent for treating sickle cell anemia?
Hydroxyurea 15–35 mg/kg/day
What are the main indications for Hydroxyurea use?
Indications:
Sickle cell anemia (≥ 3 vaso-occlusive crises per year)
Chronic myeloid leukemia
Polycythemia vera
Locally advanced squamous cell carcinoma of the head and neck (with chemoradiation)
What is the mechanism of action of Hydroxyurea?
Mechanism of Action:
Inhibits ribonucleotide reductase → blocks DNA synthesis (but not RNA or protein synthesis).
In sickle cell disease, what are the specific effects of Hydroxyurea?
Effects in Sickle Cell Disease:
Increases fetal hemoglobin (HbF)—> Decrease HbS
Reduces leukocyte and platelet counts
Decreases endothelial adhesion molecule expression (lines vessels). These combined effects help to reduce the frequency and severity of vaso-occlusive crises.
Increases nitric oxide production (NO- vasodilator)
How does Hydroxyurea improve clinical outcomes in sickle cell anemia?
Clinical Benefit:
↓ RBC polymerization
↓ Vaso-occlusion
↓ Pain crises and hospitalizations
↑ Oxygen delivery and overall outcomes
What are the major adverse effects of Hydroxyurea?
Adverse Effects:
Bone marrow suppression (leukopenia, thrombocytopenia, anemia) → requires regular CBC monitoring
Mucositis, skin ulcers, hair thinning, hyperpigmentation
GI symptoms (nausea, vomiting, diarrhea, anorexia)
Teratogenic (can harm fetus)
What are the contraindications for Hydroxyurea therapy?
Pregnancy (teratogenic)
Lactation
Severe myelosuppression
Active infection
Renal impairment (use caution)
Avoid live vaccines during therapy
What is another medication used as an oral medication for treating sickle cell anemia?
L’Glutamine (oral powder)
What are the main indications for L-Glutamine?
Indications:
Sickle cell disease (≥ 5 years old) with recurrent vaso-occlusive crises
Used as an adjunct to Hydroxyurea to further reduce crises
What is the mechanism of action of L-Glutamine in sickle cell disease?
Mechanism of Action:
L-Glutamine 🧬 → increases NAD redox ratio in sickled RBCs 🔋
→ reduces oxidative stress, RBC sickling, and adhesion to vessel walls
→ decreases frequency of vaso-occlusive pain crises 💪
How does L-Glutamine help reduce vaso-occlusive crises?
How it helps:
By restoring the redox balance and reducing oxidative damage, RBCs stay more flexible and less likely to clump, improving oxygen delivery and reducing episodes of sickling
What are the common adverse effects of L-Glutamine?
Adverse Effects:
Constipation 💩
Musculoskeletal pain 💪
Abdominal pain 🤕
Cough or headache 😷🤕
What are the contraindications for using L-Glutamine?
Contraindications:
Hypersensitivity to the drug
Severe renal or hepatic impairment
Sickle Cell Disease — Targeted Agents
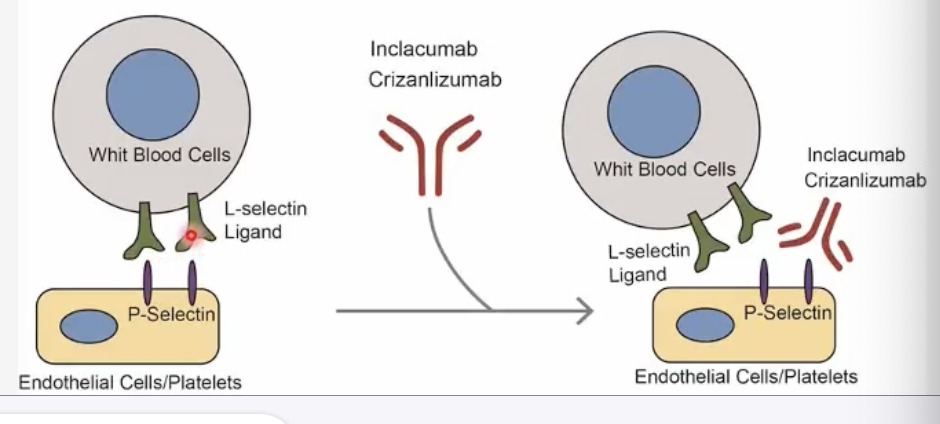
What is Crizanlizumab?
Crizanlizumab is a P-selectin inhibitor, classified as a monoclonal antibody (mAb) 💉
→ It blocks P-selectin, a cell adhesion molecule on activated platelets and endothelial cells,
→ preventing RBC and WBC adhesion → reduces vaso-occlusive crises in sickle cell disease 🩸
What are the indications for Crizanlizumab?
Indications:
Used in sickle cell disease to reduce frequency of vaso-occlusive crises
Approved for adults and pediatric patients ≥16 years old
What is the mechanism of action of Crizanlizumab?
Crizanlizumab 💉 → monoclonal antibody that binds and inhibits P-selectin (on endothelial cells & platelets)
→ P-selectin normally binds to L-selectin on WBCs, RBCs, and platelets, promoting adhesion
→ Blocking this interaction (L to P-selectin binding) prevents cells from sticking to vessel walls
→ ↓ vascular blockage (vaso-occlusion) and ↓ pain crises in sickle cell disease 🩸
What is the dosing schedule for Crizanlizumab?
Dosing Schedule:
Administered by IV infusion
5 mg/kg at Week 0, Week 2, then every 4 weeks thereafter
What are the adverse effects of Crizanlizumab?
Adverse Effects:
Infusion reactions (fever, pain) 🥵
Arthralgia (joint pain) 🤕
Nausea 🤢
Headache 😣
What is the main contraindication for Crizanlizumab use?
Contraindication:
Hypersensitivity to monoclonal antibodies 🚫
What was Voxelator (Voxelotor (oral) (HbS polymerization Inhibitor) indicated for before September 2024, when the manufacturer voluntarily withdrew voxelotor (Oxbryta) from the U.S. market due to concerns about an imbalance in vaso-occlusive crises and fatal events, and its overall benefit-risk profile is under further review.
• Sickle cell anemia (≥4 years old in US)
• Reduces transfusion dependency
A 12-year-old boy with sickle cell disease presents with 4 vaso-occlusive crises in the past year. He is started on hydroxyurea.
Question: What is the primary hematologic change induced by hydroxyurea that reduces vaso-occlusive episodes?
Hydroxyurea increases fetal hemoglobin (HbF) production 🩸
→ HbF inhibits RBC sickling → reduces polymerization, vaso-occlusion, and pain crises.
A 25-year-old man with sickle cell disease on hydroxyurea continues to have crises. He is started on an IV infusion that blocks P-selectin, reducing adhesion of blood cells to endothelium.
Question: Which drug has been prescribed?
Crizanlizumab (P-selectin inhibitor) 💉
→ Monoclonal antibody that blocks P-selectin on endothelial cells and platelets
→ Prevents adhesion of RBCs, WBCs, and platelets → decreases vaso-occlusive crises.
Name the Complement and Immune Modulators (*6)
Eculizumab (IV q2wk)/ Ravulizumab (IV q8wk)
Pegcetacoplan (subcutaneous)- Pegcetacoplan (C3 inhibitor)
Prednisone 1–2 mg/kg/day → taper- Corticosteroids
Rituximab 375 mg/m² weekly × 4—> Rituximab (Anti-CD20)
Sutimlimab- Complement Blocker (C1s inhibitor)
Cyclophosphamide, Azathioprine, Mycophenolate mofetil, Cyclosporine—> Immunosuppressive Therapy