Biology 2.1.2- Biological Molecules
1/36
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
What are the 4 main biological roles of water?
Habitat
Solvent
Coolant
Transport medium
Why is water a polar molecule?
The oxygen atom attracts the electrons more strongly than the hydrogen atoms
This gives the oxygen a weak negative charge (δ-) and the hydrogen a weak positive charge (δ+)
This means water has a dipole
How do hydrogen bonds work in water and why are they useful?
Weak hydrogen bonds form between the hydrogen and oxygen atoms of adjacent water molecules, due to it’s polarity
This means that water:
Is a good solvent as it attracts other polar molecules
Has a high specific heat capacity + latent heat of vaporisation
Is less dense when it freezes
Has a high cohesion to itself and high surface tension
How do the properties of water relate to it’s role as a solvent, and what are the examples of it?
Water is a polar molecule, so it attracts other polar molecules and dissolves them
Eg. Water can carry mineral ions in plant xylem + blood plasma carries blood cells and other substances
How do the properties of water relate to it’s role as a coolant, and what are the examples of it?
Water has a high specific heat capacity, so it can take in a lot of energy before changing temperature
It also has a high latent heat of vaporisation, so it takes in a lot of energy when boiling
Eg. Evaporation is used to cool down, by sweating or panting
How do the properties of water relate to it’s role as a habitat, and what are the examples of it?
Water has a high specific heat capacity, so it can take in a lot of energy before changing temperature
Freezes in a crystalline structure, so it is less dense when solid, meaning ice floats and can insulate water bodies
Polar molecule, so it attracts other polar molecules and can dissolve them
Eg. Creates a stable environment in ponds, with a constant temperature (for enzyme activity) and dissolved nutrients
How do the properties of water relate to it’s role as a transport molecule, and what are the examples of it?
Water is a polar molecule, so it attracts other polar molecules and can dissolve them
This also means it has high cohesion to itself and adhesion to surfaces, so can easily flow
Eg. Water can carry mineral ions in plant xylem + blood plasma carries cells and dissolved substances
What chemical elements make up carbohydrates, lipids, proteins and nucleic acids?
Carbohydrates = C, H and O
Lipids = C, H and O (+ P for phospholipids)
Proteins = C, H, O and N (+ P and S sometimes)
Nucleic acids = C, H, O, N and P
What are the biological cations calcium, sodium, potassium, hydrogen and ammonium each used for?
Calcium (Ca 2+) - nerve impulses and muscle contractions
Sodium (Na +) - nerve impulses, transport of substances across cell membranes and kidney function
Potassium (K +) - nerve impulses, kidney function and stomata
Hydrogen (H +) - catalysts and pH determination
Ammonium (NH4 +) - used in protein synthesis
What are the biological anions nitrate, hydrogen carbonate, chloride, phosphate and hydroxide each used for?
Nitrate (NO3 -) - amino acid formation
Hydrogen carbonate (HCO3 -) - maintains blood pH
Chloride (Cl -) - balance sodium and potassium ions in cells and maintains blood pH
Phosphate (PO4 3-) - cell membranes, bone formation, and is a component of DNA, RNA and ATP
Hydroxide (OH -) - catalysts and pH determination
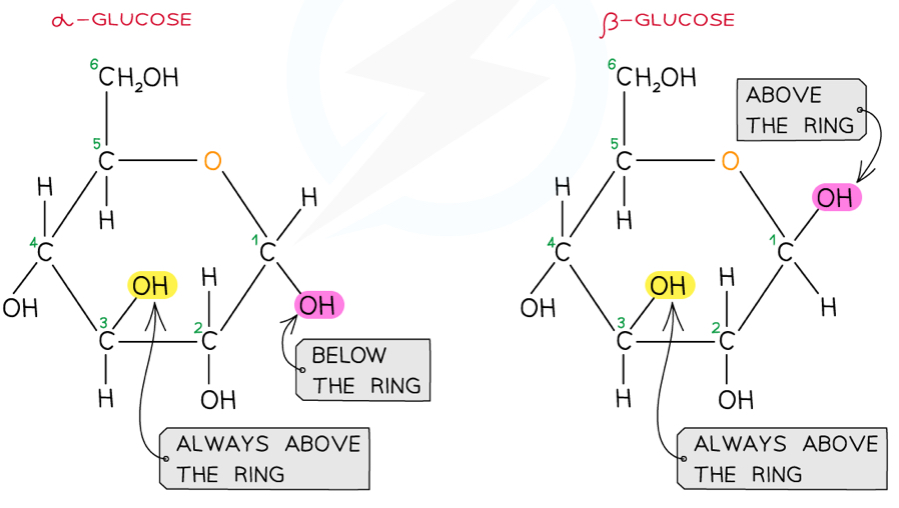
What are the structures of alpha and beta glucose?
Glucose is a hexose sugar with two isomers- they both have the formula C6H12O6
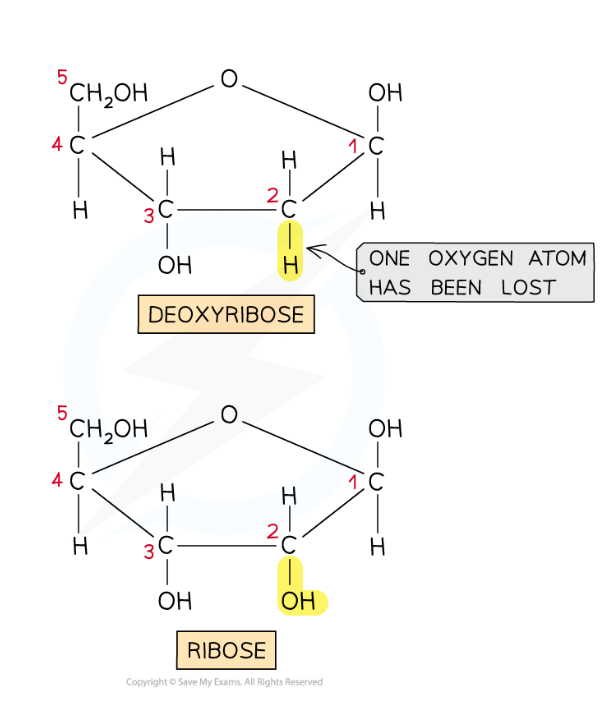
What are the structures of ribose and deoxyribose?
Ribose and deoxyribose are pentose (5 carbon) sugars, with similar formulas except that deoxyribose has one less oxygen than ribose (lost from the second carbon)
What three properties do monosaccharides have in common?
Soluble in water
Sweet tasting
Forms crystals
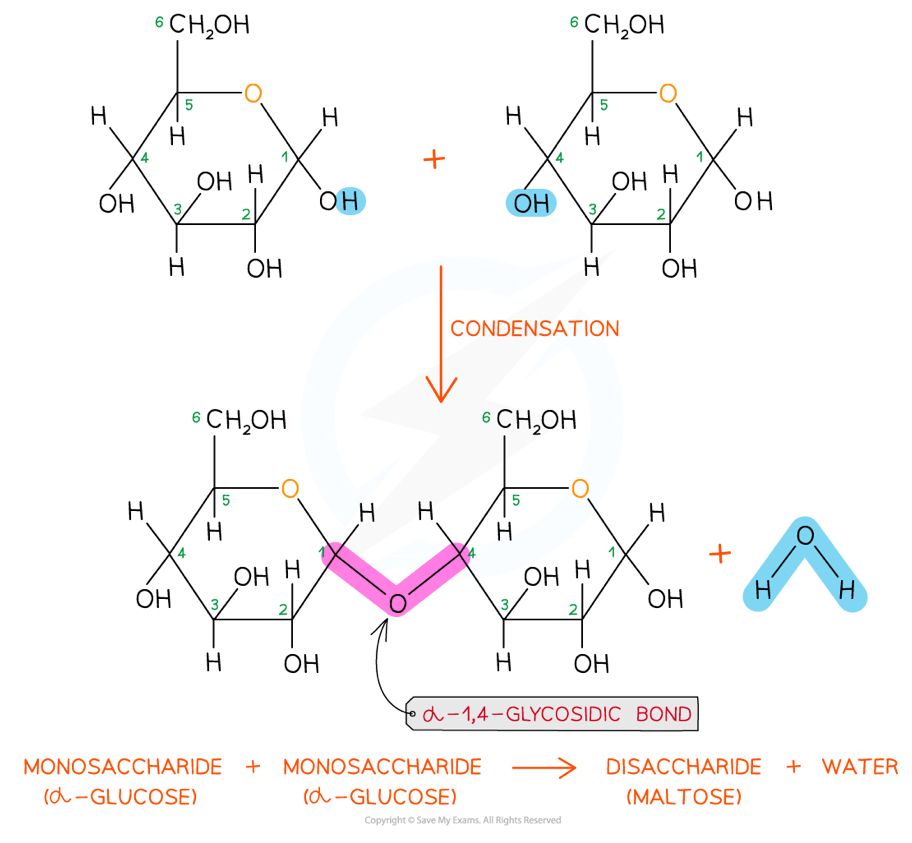
How can disaccharides and polysaccharides be formed and broken down?
They can be formed by condensation reactions- when two hydroxyl (OH) groups from different saccharides interact to produce a water molecule and a glycosidic bond between the two saccharides
This can be catalysed by enzymes
They can be broken down by hydrolysis- when water is added to a di or polysaccharide, breaking the glycosidic bond to form a hydroxyl group on each saccharide
This can be catalysed by (different) enzymes
We use this to test for non reducing sugars
What are the three most common disaccharides made from?
Maltose- two glucose molecules
Sucrose- glucose + fructose
Lactose- glucose + galactose
What are reducing sugars?
Reducing sugars can give away electrons via the oxidisation of a carbonyl (C=O) group
This is why reducing sugars can be detected using Benedict’s solution- they reduce the soluble blue copper sulphate to insoluble brick-red copper oxide
All monosaccharides and some disaccharides are reducing sugars- polysaccharides aren’t
How can we detect non-reducing sugars and why?
Non-reducing sugars (di or poly saccharides) must be broken down into their monosaccharides, which are always reducing sugars, to be detected using Benedict’s solution
We do this by hydrolysis, where we heat the sample with hydrochloric acid to break the glycosidic bond, and then neutralise it
Then we can test with Benedict’s solution to see whether reducing sugars were produced, and hence whether non reducing sugars were originally present
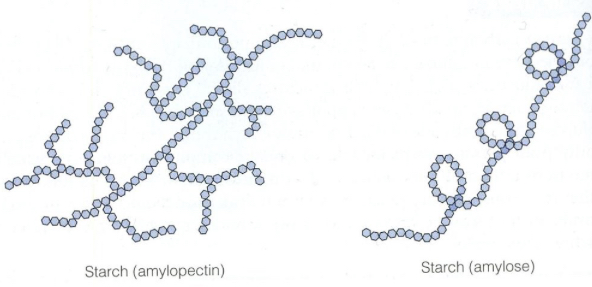
What is the structure of starch?
Starch is made from two different alpha glucose structures :
Amylose (20%)- a straight chain linked by 1,4-glycosidic bonds- amylose curls into a helix shape which allows it to be more compact
Amylopectin (80%)- a branched chain linked by 1,4 and 1,6-glycosidic bonds
What is starch used for and how is it well suited?
Starch is the main carbohydrate store in plants
Stored in the plastids- amyloplasts and chloroplasts
This because it is:
Compact, so large quantities can be stored
Insoluble, so it won’t change the water concentration in cells and affect osmosis
Amylopectin (80%) is linked by some 1,6-glycosidic bonds, so it has many terminal glucose molecules that can be hydrolysed for respiration or added for storage
What is the structure of glycogen?
Made up of alpha glucose molecules linked by 1,6 and 1,4-glycosidic bonds
Glycogen has a similar structure to amylopectin but is more branched, because it has more 1,6-bonds
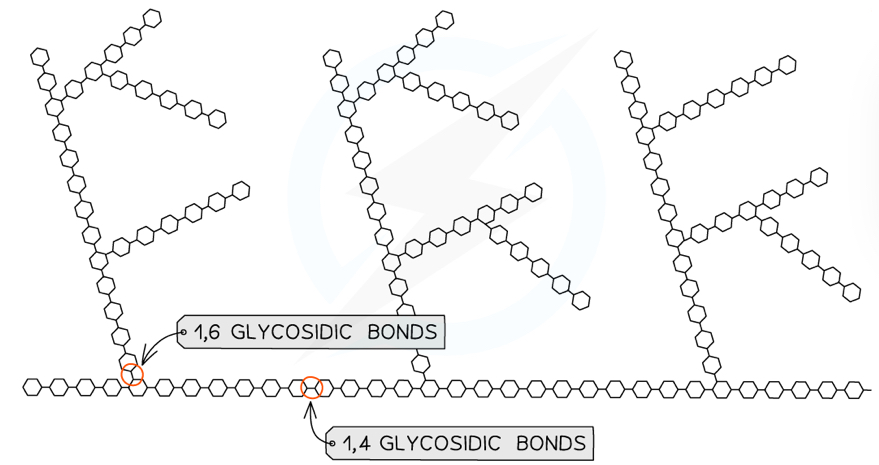
What is glycogen used for and how is it well suited?
Glycogen is used for storage in animals
Stored in liver and muscle cells
This because it is:
Compact but relatively large, so large quantities can be stored (more 1,6-bonds means it is more compact than amylopectin)
Insoluble, so it won’t change the water concentration in cells and affect osmosis
Linked by many 1,6-glycosidic bonds so it has many terminal glucose molecules that can be hydrolysed for respiration or added for storage
What is the structure of cellulose?
Made up of beta glucose molecules linked by 1,4-glycosidic bonds
To bond together, every other beta glucose molecule is flipped
This means that hydrogen bonds can form between strands, to create microfibrils
These make up the cellulose fibres that link into a network
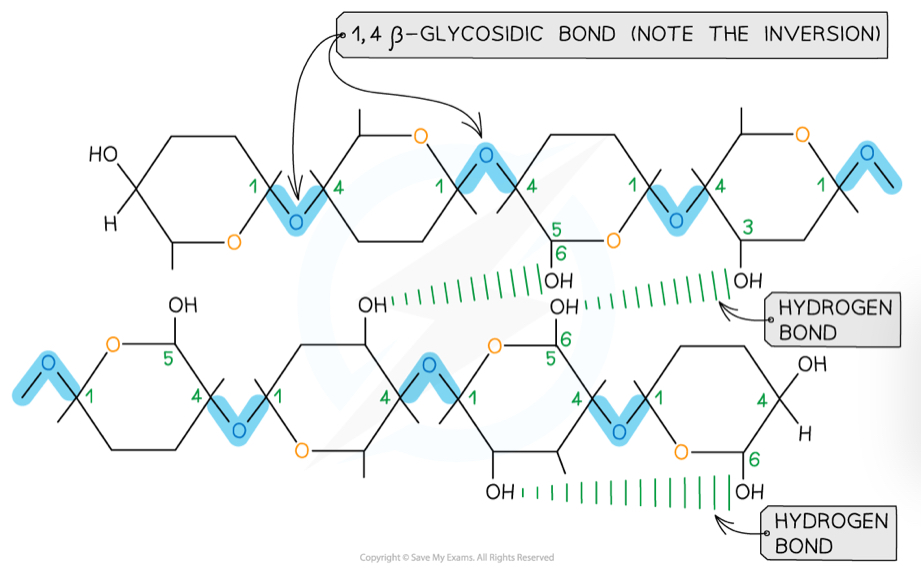
What is cellulose used for and how is it well suited?
Cellulose makes up the majority of plant cell walls
This is because it is:
Held together by many hydrogen bonds between strands, so it has a very high tensile strength and is able to withstand the pressure from turgidity of the cell
Linked to other molecules like lignin, which increases the strength of the cell walls
Permeable, so water and solutes can enter or leave the cell
What are triglycerides?
Triglycerides are lipids made up of a glycerol backbone bonded with three fatty acid chains
The three hydroxyl groups on glycerol and the carboxyl group on each fatty acid go through a condensation reaction to create ester bonds (process of esterification)
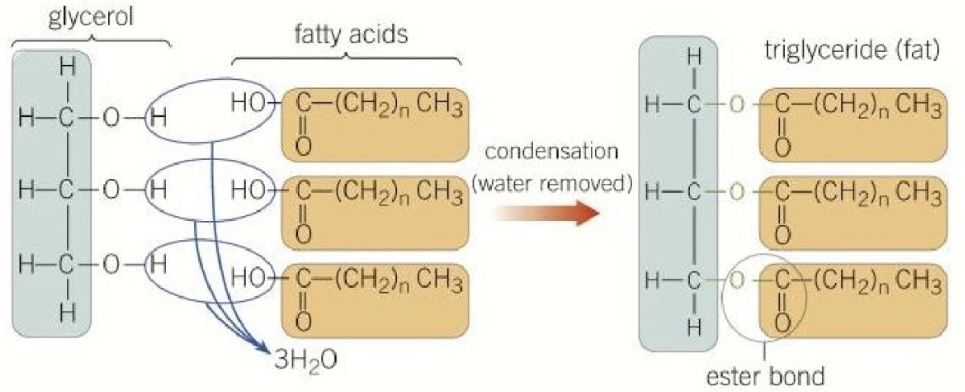
How can triglycerides differ in structure?
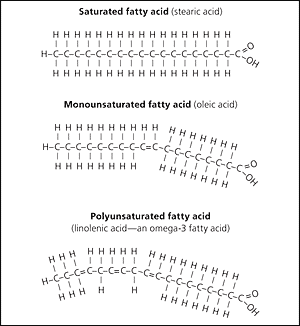
Fatty acids can differ in length
Fatty acids can be saturated (all single bonds) or unsaturated (containing a double bond)
A mono-unsaturated fatty acid has one double bond, while a poly-unsaturated one has multiple
The double bonds in an unsaturated fatty acid cause the molecule to bend, so they can’t pack together as closely, and they are liquids (oils)
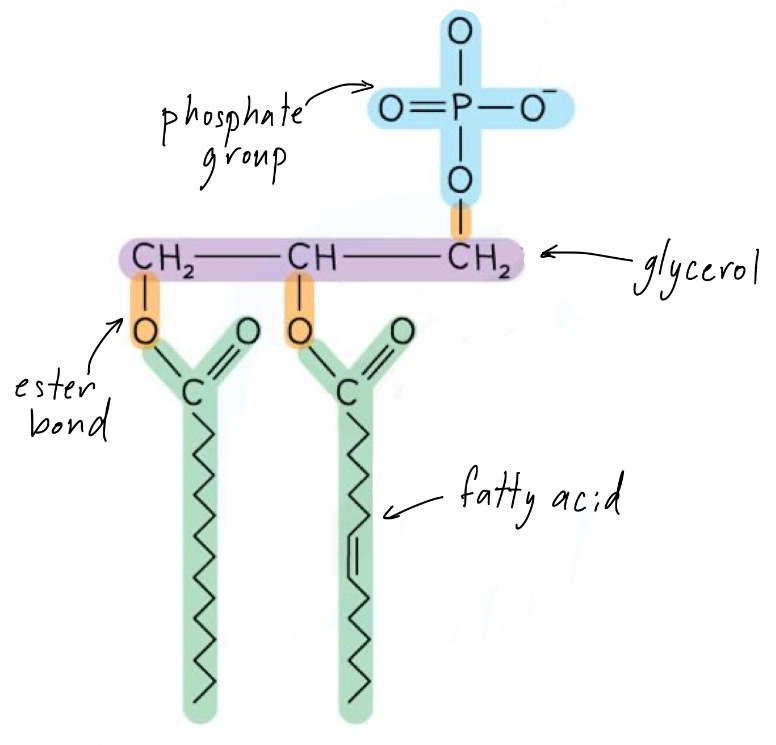
What are phospholipids?
Phospholipids are lipids made up of a glycerol backbone joined to two fatty acids and a phosphate group
The phosphate group is polar, so it is hydrophilic (attracted to water and aqueous solutions)
Whereas the fatty acids are non-polar and hydrophobic
This allows phospholipids to form cell membranes
How do the properties of triglycerides relate to their function?
Triglycerides are mainly used as energy storage molecules, because:
They’re insoluble, so they don’t affect osmosis in cells and can be stored in large quantities
The long fatty acid chains contain lots of chemical energy (more than carbohydrates) so triglycerides can store energy and release it when broken down
Plants mostly store unsaturated fats, while animals saturated fats
Triglycerides are also used to insulate nerve fibres in the myelin sheath and insulate animals against heat loss (as part of the adipose tissue layer), provide buoyancy and protect organs
How do the properties of phospholipids relate to their function?
Phospholipids make up cell membranes, because:
The phosphate group is hydrophilic and the fatty acids are hydrophobic, so they can form a bilayer with the phosphate groups facing aqueous solution and the fatty acids in the middle
This allows cells to regulate the concentration of substances and compartmentalise organelles, improving efficiency
How do the properties of cholesterol relate to it’s function?
Cholesterol strengthens and stabilises the phospholipid membrane, because:
They are small and flat molecules, so can fit between phospholipid molecules
They bond to the hydrophobic fatty acids, packing the phospholipids in more closely and rigidly- this decreases the fluidity of the cell membrane
Cholesterol is only found in animal cell membranes
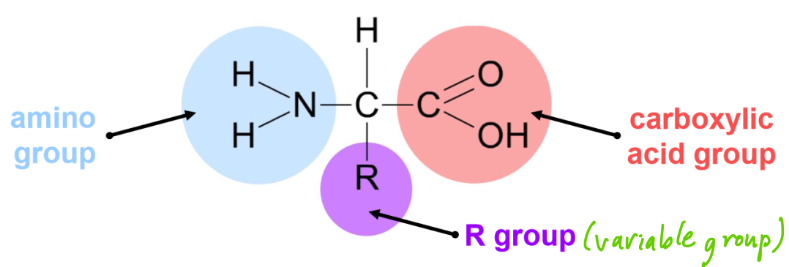
What is the structure of amino acids?
There are 20 amino acids found in proteins- these differ by having different R groups
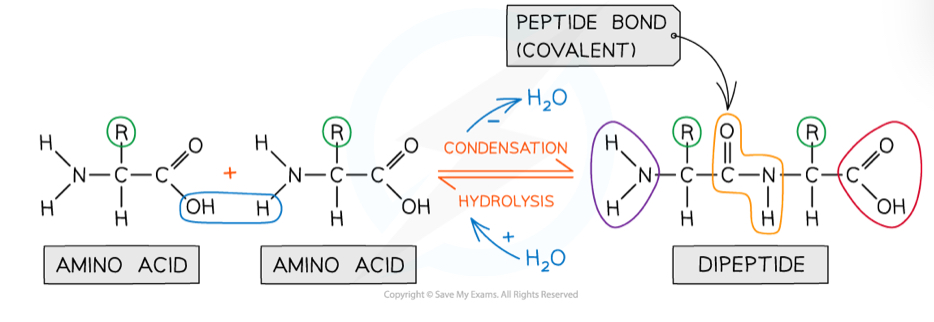
How are dipeptides and polypeptides formed?
A peptide bond between two amino acids is formed through condensation- when the OH from the carboxyl group of one amino acid reacts with an H from the amine group of another amino acid
Peptide bonds are covalent
This is reversed by a hydrolysis reaction
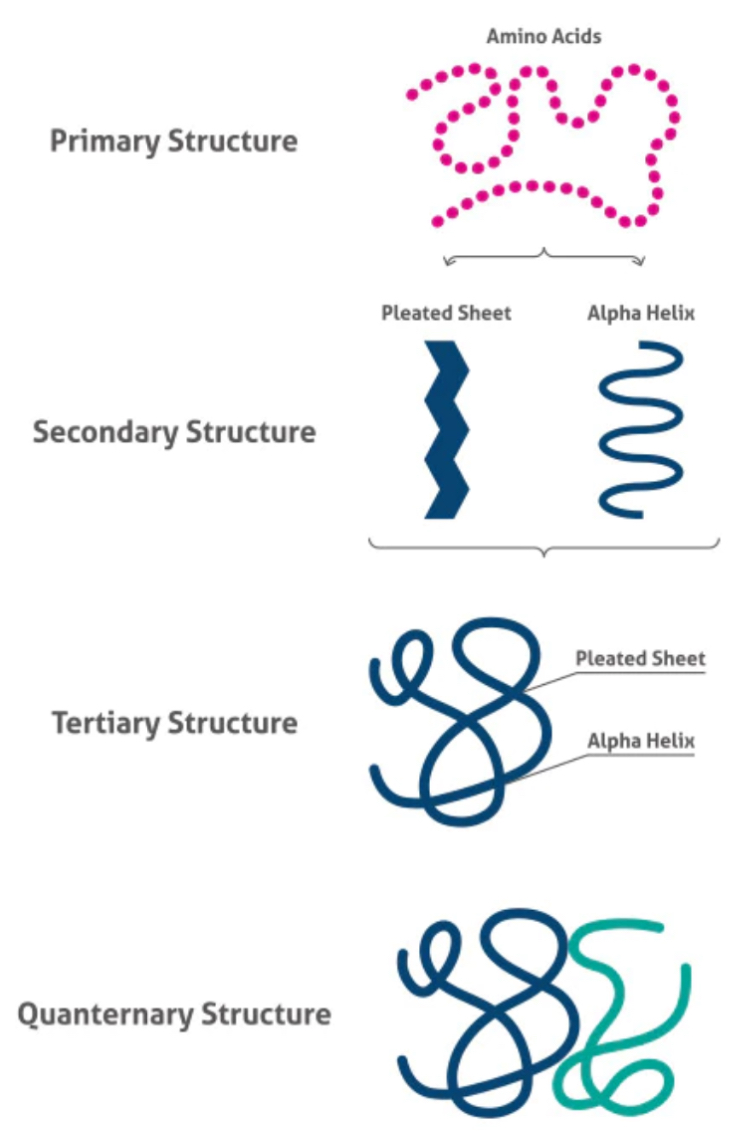
What are the levels of protein structure?
Primary- the sequence of amino acids in the polypeptide chain
Secondary- how hydrogen bonds between amine groups and carboxyl groups cause the chain to fold into a beta pleated sheet or coil into an alpha helix
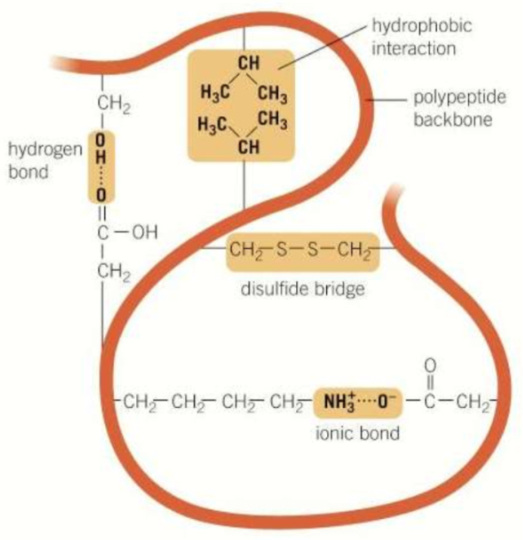
Tertiary- hydrophilic/phobic interactions, hydrogen bonds, ionic bonds and disulphide bonds hold the R groups together into a complicated shape
Quaternary- multiple polypeptide chains (subunits) joined together, eg. in haemoglobin
Compare the strengths of different bonds within the tertiary structure of a protein
Strongest- disulphide bridges + ionic bonds
Middle- hydrogen bonds
Weakest- hydrophobic + hydrophilic interactions
Describe the structure and function of globular proteins
Globular proteins form a spherical shape because their hydrophobic R groups are orientated towards the centre of the protein but their hydrophilic R groups are positioned on the outside of the protein
This means that most globular proteins are soluble
The amount of possible tertiary structures of globular proteins gives them very specific shapes (beneficial for enzymes and antibodies)
Globular proteins can be conjugated by containing a prosthetic group eg. haemoglobin
Globular proteins are transport molecules, enzymes, and hormones
Describe an example of each of the three functions of globular proteins
Haemoglobin is a transport molecule found in red blood cells
It is made up of four polypeptide chains and four prosthetic haem groups (it is a conjugated protein), which are able to temporarily bond to oxygen
Oxygen is not very soluble in water but haemoglobin is soluble, so it allows oxygen to be carried more efficiently around the body for respiration
Enzymes, eg. amylase and catalase, are biological catalysts that speed up reactions in the body
There are many possible structures of globular proteins due to bonding between R groups, which allows enzymes to have very specifically shaped active sites complementary to their function
Insulin is a hormone involved in the regulation of blood glucose concentration
Hormones are transported in the bloodstream so they need to be soluble
Hormones have to have a specific shape to activate receptors, which is possible due to the variations in globular protein structure
Describe the structure and function of fibrous proteins
Fibrous proteins are made up of parallel polypeptide chains held together by cross links, forming long, rope-like fibres
They have many hydrophobic R groups, making them insoluble
Fibrous proteins have a limited number of amino acids with the sequence usually being highly repetitive
This forms organised structures that are very strong
Fibrous proteins are used as structural components
Describe the three main fibrous proteins
Keratin is found in hair, skin and nails
It contains lots of disulphide bridges, which makes it strong, inflexible and insoluble
Elastin is found in elastic fibres like the walls of blood vessels and the skin
It is a quaternary protein made from chains of a stretchy polypeptide, which makes it insoluble, stable and elastic
Collagen is a structural protein forming connective tissues
Collagen molecules have a triple helix structure held together by hydrogen bonds, giving it a high tensile strength