x ray beam
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
88 Terms
![<p>(Milliamperage)(time) --> mAs</p><ul><li><p>mA is a user selectable control (sometimes it can be constant)<br></p><ul><li><p>if constant, <span><strong>[what's the problem?]</strong></span></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f6c871b0-0ffc-4903-af2b-281d9fd9882b.png)
(Milliamperage)(time) --> mAs
mA is a user selectable control (sometimes it can be constant)
if constant, [what's the problem?]
patient motion is a risk that it will blur the image
![<p>(Milliamperage)(time) --> mAs</p><ul><li><p>Milliamperage is <span><strong>[...]</strong></span></p></li><li><p>mAs is <span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ff96b6d4-fe83-42c9-9077-50dbe5883a6f.png)
(Milliamperage)(time) --> mAs
Milliamperage is [...]
mAs is [...]
quantity of photons
the total number of photons/radiations during a specific time of exposure
![<p>15% change in Kilovoltage</p><ul><li><p>Quality of photonic energy is <span><strong>[...]</strong></span></p></li><li><p>Quantity of photons is also <span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/5b4b52e5-b0ec-4bc5-a620-10af9999801b.png)
15% change in Kilovoltage
Quality of photonic energy is [...]
Quantity of photons is also [...]
increased (peak is further down)
increased (increased area under the curve)
![<p>15% Rule</p><ul><li><p>In order to maintain image density, an increase of kVp by 15% should be accompanied by a <span><strong>[...]</strong></span>% <span><strong>[increase or decrease]</strong></span> of mAs</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/67bb5d6c-c03a-4447-97c6-3e6a745467e9.png)
15% Rule
In order to maintain image density, an increase of kVp by 15% should be accompanied by a [...]% [increase or decrease] of mAs
50% decrease
Inc. in 15% of kVp = dec. in 50% of mAs
![<p>4 basic factors affecting quantity of x-ray beam photons</p><ol><li><p><span><strong>[...]</strong></span></p></li><li><p><span><strong>[...]</strong></span></p></li><li><p><span><strong>[...]</strong></span></p></li><li><p><span><strong>[...]</strong></span> </p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f6958377-143d-41ec-9652-80adc6de204c.png)
4 basic factors affecting quantity of x-ray beam photons
[...]
[...]
[...]
[...]
(Milliamperage)(time) --> mAs
Kilovoltage (kVp)
Distance
Filtration
Absorbed Dose (D)
Conversion
One gray = [...] Joule (J) of energy deposited per kilogram
One rad = [...] ergs of energy deposited per gram
1 Gy = [...] Rads
1 rad = [...] mGy
1
100
100
10
Absorbed Dose (D)
D = [...]
Units:
SI system – Gray (Gy)
Non-SI – Rads
E/M
Absorbed Dose (D)
D = E/M
Units:
SI system – [...]
Non-SI – [...]
Gray (Gy)
Rads
![<p><span>As a polyenergetic beam goes through objects, how does it impact the HVL? </span></p><ul><li><p><span><strong>[...]</strong></span> </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b7cef675-0fe5-409e-ba11-cb468e44920e.png)
As a polyenergetic beam goes through objects, how does it impact the HVL?
[...]
Since the beam is getting more energetic, HVL increases after each obstacle because the thickness needed to decrease beam intensity has to go up when beam intensity is increasing
Basic interactions with matter
Insignificant
[...]
[...]
[...]
Significant
Photoelectric absorption
Compton Scatter
Coherent scatter (5%)
Pair Production
Photodisintegration
Basic interactions with matter
Insignificant
Coherent scatter (5%)
Pair Production
Photodisintegration
Significant
[...]
[...]
Photoelectric absorption
Compton Scatter
Basics of Attenuation
Factors increasing attenuation
[...]
[...]
[...]
Density
Atomic Number (Z)
Electrons per gram of tissue
Density = denser objects will decrease intensity and therefore increase attenuation
Atomic Number (Z) = higher atomic numbers present more obstacles (more electrons, protons, etc.) and therefore increase attenuation
Electrons per gram of tissue
![<p>Compton Scatter Angle of deflection</p><ul><li><p>Greater the angle, the <span><strong>[more or less]</strong></span> energy is lost </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/60ddb821-56a4-4c98-a3c8-12ae8c8157a5.png)
Compton Scatter Angle of deflection
Greater the angle, the [more or less] energy is lost
more
![<p><span>Does an x-ray beam have more or less energy after it goes through a wall?</span></p><ul><li><p><span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/98e0f41d-3d1f-4240-af5d-14c30d597743.png)
Does an x-ray beam have more or less energy after it goes through a wall?
[...]
An x-ray beam that makes it through a wall has more energy because only the strong beams make it through the wall
![<p><span>Does changing the kilovoltage affect the quality? </span></p><ul><li><p><span><strong>[...]</strong></span></p></li><li><p><span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/63420237-ac22-4ba0-9278-97eb233be4b7.png)
Does changing the kilovoltage affect the quality?
[...]
[...]
yes, overall effect on film blackening is approximately equal to the fourth power
peak is moved to the right a little
![<p><span>Does doubling the Milliamperage change energy distribution?</span></p><ul><li><p><span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/55d15308-892a-4be4-93b4-ccaedeea0a2b.png)
Does doubling the Milliamperage change energy distribution?
[...]
does NOT change energy distribution (peak is at the same spot)
Dose Equivalent (H)
H = [...] x [...]
absorbed dose x quality factor
H = D x QF
Dose Equivalent (H)
Quality factor – derived from LET values
QF of x-rays, Gamma, electrons, beta particles is [...]
So, 1 rad = [...] rem in diagnostic radiology
QF may be as high as 20 for alpha particles/heavy nuclei
1.0
1
Dose Equivalent (H)
Units for Dose Equivalent (H)
SI system – [...]
Non- SI – [...]
Conversion rates
1 Sv = 100 rem
1 rem = 10 mSv
Sievert (Sv)
Rem (rad. Equiv. man)
Dose Equivalent (H)
Units for Dose Equivalent (H)
SI system – Sievert (Sv)
Non- SI – Rem (rad. Equiv. man)
Conversion rates
1 Sv = [...] rem
1 rem = [...] mSv
100
10
![<p>Doubling Filter Thickness</p><ul><li><p><span><strong>[increase or decrease]</strong></span> quality</p></li><li><p><span><strong>[increase or decrease]</strong></span> quantity</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/a0bae12e-ed54-4d7a-9e2f-282d17f1b3bd.png)
Doubling Filter Thickness
[increase or decrease] quality
[increase or decrease] quantity
Increases
Decreases
![<p>Doubling Filter Thickness</p><ul><li><p><span><strong>[how does it affect quality?]</strong></span></p></li><li><p><span><strong>[how does it affect quantity?]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/9eca1f87-d957-45e1-b44f-fdb5d19b972e.png)
Doubling Filter Thickness
[how does it affect quality?]
[how does it affect quantity?]
Rightward shift of the peak which means there is a higher quality of energy produced (bc lower energy x-rays are filtered out)
Area under the curve decreases because the quantity of the energy decrease (bc some of the photons are obviously being eliminated)
![<p>Effect of Milliamperage Doubling</p><ul><li><p><span><strong>[what happens to quality?]</strong></span></p></li><li><p><span><strong>[what happens to quantity?]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f5551a69-da2e-45a0-a15c-0f3768222641.png)
Effect of Milliamperage Doubling
[what happens to quality?]
[what happens to quantity?]
Quality of photonic energy is NOT affected (peak is at the same spot)
Quantity of photons double (area under the curve is doubled)
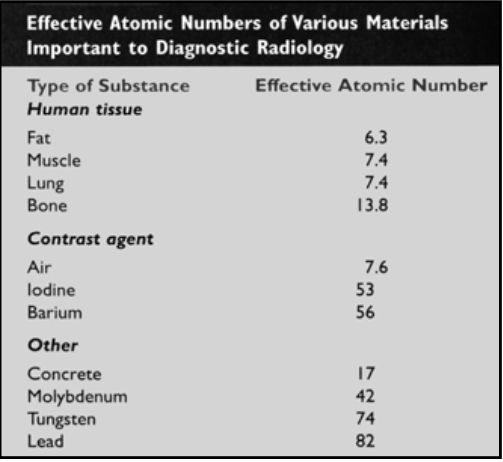
![<p>Effective Atomic Numbers of Various Materials Important to Diagnostic Radiology</p><ul><li><p>Contrast Agents = <span><strong>[...]</strong></span>, <span><strong>[...]</strong></span>, <span><strong>[...]</strong></span><br></p><ul><li><p>Helps to have clearer images</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/cbe6bdd7-58b7-427a-b5c1-2c54d4b26ac3.png)
Effective Atomic Numbers of Various Materials Important to Diagnostic Radiology
Contrast Agents = [...], [...], [...]
Helps to have clearer images
Air, Iodine, Barium
![<p>Effective Atomic Numbers of Various Materials Important to Diagnostic Radiology</p><ul><li><p>Human tissue (fat, muscle, lung, bone)<br></p><ul><li><p><span><strong>[what has the highest effective atomic number]</strong></span></p></li><li><p>Overall <span>low</span> effective atomic numbers</p><ul><li><p><span>Indicates a low BE that is so low that none of the characteristic radiation will exit the body but will rather be absorbed</span> </p></li></ul></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/16359786-913a-433c-96ce-19a890791a84.png)
Effective Atomic Numbers of Various Materials Important to Diagnostic Radiology
Human tissue (fat, muscle, lung, bone)
[what has the highest effective atomic number]
Overall low effective atomic numbers
Indicates a low BE that is so low that none of the characteristic radiation will exit the body but will rather be absorbed
Bone has the highest effective atomic number
![<p>Effective Atomic Numbers of Various Materials Important to Diagnostic Radiology</p><ul><li><p>Human tissue (fat, muscle, lung, bone)<br></p><ul><li><p><span>Bone has the highest effective atomic number</span></p></li><li><p>Overall <span><strong>[high or low]</strong></span> effective atomic numbers</p><ul><li><p><span><strong>[what does this indicate about absorption?]</strong></span> </p></li></ul></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ad490713-1d9e-46bc-b4a2-f69c772f6353.png)
Effective Atomic Numbers of Various Materials Important to Diagnostic Radiology
Human tissue (fat, muscle, lung, bone)
Bone has the highest effective atomic number
Overall [high or low] effective atomic numbers
[what does this indicate about absorption?]
low
Indicates a low BE that is so low that none of the characteristic radiation will exit the body but will rather be absorbed
Factors decreasing attenuation
[...]
Kilovoltage
increasing kVp will just increase punching power so it will just break through matter like hulk
![<p><span>How are beam intensity and mA related?</span></p><ul><li><p><span><strong>[...]</strong></span> </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/266608d3-606e-489f-a1ce-6595adb011b6.png)
How are beam intensity and mA related?
[...]
Beam intensity and mAs are directly proportional
Doubling mAs will double the number of emitted x-rays
HVL [increases or decreases] with increasing filtration
increases
High-Value Layer: thickness of an aluminum absorber that is required to reduce beam intensity by 50%
HVL increases with [increased or decreased] filtration
increasing
High-Value Layer: thickness of an aluminum absorber that is required to reduce beam intensity by 50%
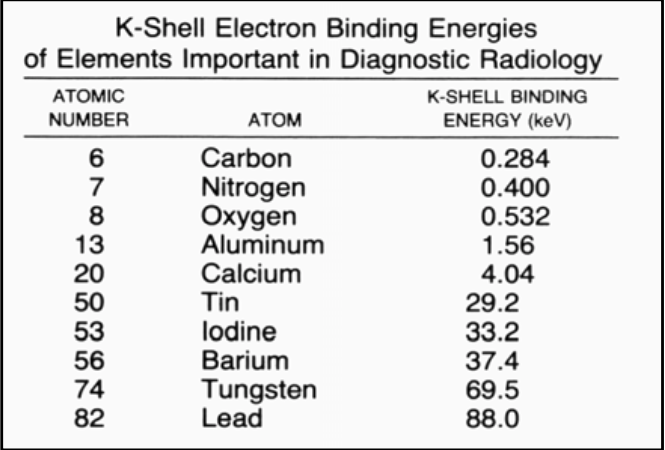
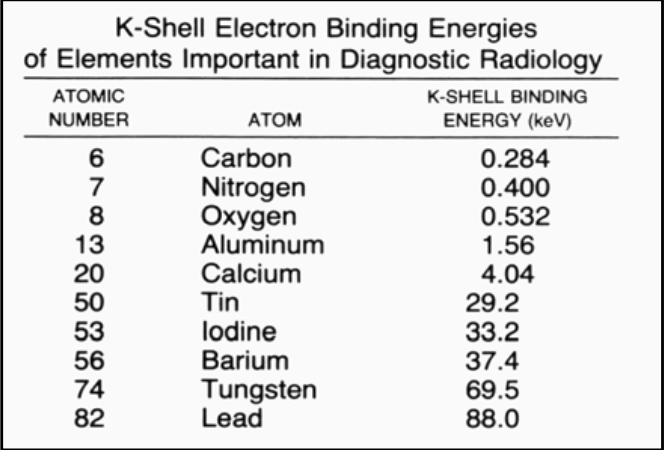
![<p>If you want to use the barium as the contrast agent, what should the x-ray kVp be set as? </p><ul><li><p><span><strong>[...]</strong></span> </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/d585369b-0cc4-46b5-ab55-d431df9ac412.png)
If you want to use the barium as the contrast agent, what should the x-ray kVp be set as?
[...]
Barium = 37.4 K-shell binding energy. So, set the x-ray machine to about 112 so that the peak will be at about 37.4 (1/3 of the total) and therefore allowing a photoelectric reaction
![<p>Intensity (exposure) = product of number of photons & their average energy</p><ul><li><p>Intensity = (<span><strong>[...]</strong></span>)(<span><strong>[...]</strong></span>)</p></li><li><p>Unit of measurement = Roentgen (R)<br></p><ul><li><p>1 R = <span>amount of radiation to liberate 2.58e-4 Coulombs per kg of air</span></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/8c5664bd-1fff-420e-8720-9aeb49c54358.png)
Intensity (exposure) = product of number of photons & their average energy
Intensity = ([...])([...])
Unit of measurement = Roentgen (R)
1 R = amount of radiation to liberate 2.58e-4 Coulombs per kg of air
(quantity)(quality)
![<p>Intensity (exposure) = product of number of photons & their average energy</p><ul><li><p>Intensity = (<span>quantity</span>)(<span>quality</span>)</p></li><li><p>Unit of measurement = Roentgen (R)<br></p><ul><li><p>1 R = <span><strong>[...]</strong></span></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ab3dd700-df52-445f-b4ae-b8f19575784f.png)
Intensity (exposure) = product of number of photons & their average energy
Intensity = (quantity)(quality)
Unit of measurement = Roentgen (R)
1 R = [...]
amount of radiation to liberate 2.58e-4 Coulombs per kg of air
![<p><span>Inverse Square Law</span></p><ul><li><p>Doubling distance from x-ray source <span><strong>[increases or decreases]</strong></span> intensity by a factor of <span><strong>[...]</strong></span> </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/9a6a0d19-d2bd-49b4-a378-367558a723ab.png)
Inverse Square Law
Doubling distance from x-ray source [increases or decreases] intensity by a factor of [...]
decreases
4
Radiation intensity varies inversely with the distance squared from the source
SAME number of photons, just diluted with distance
![<p><span>Is it safer to increase the distance between you and the x-ray beam or is lead better? </span></p><ul><li><p><span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/612c95c3-ec1b-48e3-bfc2-e77514815407.png)
Is it safer to increase the distance between you and the x-ray beam or is lead better?
[...]
distance
Linear Energy Transfer (LET)
High LET has [high or low] penetration but [high or low] absorption
Low LET has [high or low] penetration but [high or low] absorption
Low, High
High, low
Linear Energy Transfer (LET)
[directly or inversely] related to particle KE
Inversely
Linear Energy Transfer (LET)
[high or low] LET = photons electrons, gamma, & x-rays
[high or low] LET = neutrons, protons, and alpha particles
low
high
Lower HVL means the beam has [...]
too many low-beam photons
High-Value Layer: thickness of an aluminum absorber that is required to reduce beam intensity by 50%
HVL increases with filtration, so a low HVL doesn't have much filtration which means a lot of low energy photon gets through
![<p>Monoenergetic Attenuation</p><ul><li><p><span><strong>[how does a monoenergetic beam respond to more HVL layers?]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/9dd58b51-e9e7-4acb-811d-77f7c2171183.png)
Monoenergetic Attenuation
[how does a monoenergetic beam respond to more HVL layers?]
With more HVL layers, a monoenergetic beam decreases in quantity (number of photons)
With more HVL layers, a monoenergetic beam decreases in quantity (number of photons)
nearly the same
![<p>PE vs Compton Scatter Probabilities</p><ul><li><p>Relative probabilities<br></p><ul><li><p>Almost everything we see in imaging is due to <span><strong>[PE or Compton]</strong></span> because they’re what is present in high x-ray energies</p></li><li><p>Safety factor: x-ray machine with high kVp potential usually require lead placed into the wall as a precaution, because <span>you don’t know where the electrons will scatter</span></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/0dd0f4d3-38d4-4a90-99f5-71427adf067e.png)
PE vs Compton Scatter Probabilities
Relative probabilities
Almost everything we see in imaging is due to [PE or Compton] because they’re what is present in high x-ray energies
Safety factor: x-ray machine with high kVp potential usually require lead placed into the wall as a precaution, because you don’t know where the electrons will scatter
Compton scatters
![<p>PE vs Compton Scatter Probabilities</p><ul><li><p>Relative probabilities<br></p><ul><li><p>Almost everything we see in imaging is due to <span>Compton scatters</span> because they’re what is present in high x-ray energies</p></li><li><p>Safety factor: x-ray machine with high kVp potential usually require lead placed into the wall as a precaution, because <span><strong>[...]</strong></span></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3b2578ab-452d-4f34-b3b2-e83b688dafcd.png)
PE vs Compton Scatter Probabilities
Relative probabilities
Almost everything we see in imaging is due to Compton scatters because they’re what is present in high x-ray energies
Safety factor: x-ray machine with high kVp potential usually require lead placed into the wall as a precaution, because [...]
you don’t know where the electrons will scatter
![<p>PE vs Compton Scatter Probabilities</p><ul><li><p>With water<br></p><ul><li><p>Photoelectric reactions increase with <span><strong>[increase or decreased]</strong></span> kVp</p></li><li><p>Compton Scatter reactions increase with <span><strong>[increase or decreased]</strong></span> kVp </p></li><li><p>At <span>26</span> kVp, 50% of interactions are photoelectric and Compton scatter </p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b000c5f0-76b5-4712-8072-eefc4c1884a5.png)
PE vs Compton Scatter Probabilities
With water
Photoelectric reactions increase with [increase or decreased] kVp
Compton Scatter reactions increase with [increase or decreased] kVp
At 26 kVp, 50% of interactions are photoelectric and Compton scatter
decreased
increased
Meaning at low kVp, our bodies are significantly absorbing radiation
![<p>PE vs Compton Scatter Probabilities</p><ul><li><p>With water<br></p><ul><li><p>Photoelectric reactions increase with <span>decreased</span> kVp</p></li><li><p>Compton Scatter reactions increase with <span>increased</span> kVp </p></li><li><p>At <span><strong>[...]</strong></span> kVp, 50% of interactions are photoelectric and Compton scatter </p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/031d1522-575d-47e7-8b7a-5972834b8bd3.png)
PE vs Compton Scatter Probabilities
With water
Photoelectric reactions increase with decreased kVp
Compton Scatter reactions increase with increased kVp
At [...] kVp, 50% of interactions are photoelectric and Compton scatter
26
Meaning at low kVp, our bodies are significantly absorbing radiation
![<p>PE vs Compton Scatter Probabilities</p><ul><li><p><strong>With bone</strong><br></p><ul><li><p>Photoelectric reactions increase with <span><strong>[increased or decreased]</strong></span> kVp</p></li><li><p>Compton Scatter reactions increase with <span><strong>[increased or decreased]</strong></span> kVp</p></li><li><p>At <span>45</span> kVp, 50% of interactions are photelectric and Compton scatter</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/6704cd79-00ee-498f-a37b-2ef1f647369d.png)
PE vs Compton Scatter Probabilities
With bone
Photoelectric reactions increase with [increased or decreased] kVp
Compton Scatter reactions increase with [increased or decreased] kVp
At 45 kVp, 50% of interactions are photelectric and Compton scatter
decreased
increased
![<p>PE vs Compton Scatter Probabilities</p><ul><li><p><strong>With bone</strong><br></p><ul><li><p>Photoelectric reactions increase with <span>decreased</span> kVp</p></li><li><p>Compton Scatter reactions increase with <span>increased</span> kVp</p></li><li><p>At <span><strong>[...]</strong></span> kVp, 50% of interactions are photelectric and Compton scatter</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/4c22068a-85ca-4c02-a2f6-44f3e5ab047c.png)
PE vs Compton Scatter Probabilities
With bone
Photoelectric reactions increase with decreased kVp
Compton Scatter reactions increase with increased kVp
At [...] kVp, 50% of interactions are photelectric and Compton scatter
45
![<p>Photoelectric Reaction</p><ul><li><p>(<span><strong>[...]</strong></span>) – (<span><strong>[...]</strong></span>) = KE of the electron that escapes</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3a45dfcc-2fbd-4f70-9b33-8739db3b43a1.png)
Photoelectric Reaction
([...]) – ([...]) = KE of the electron that escapes
(Energy of the incident photon) – (electron BE)
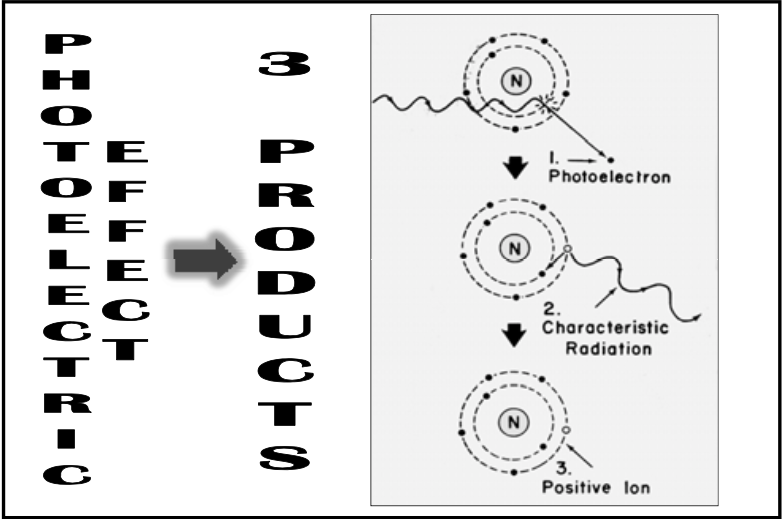
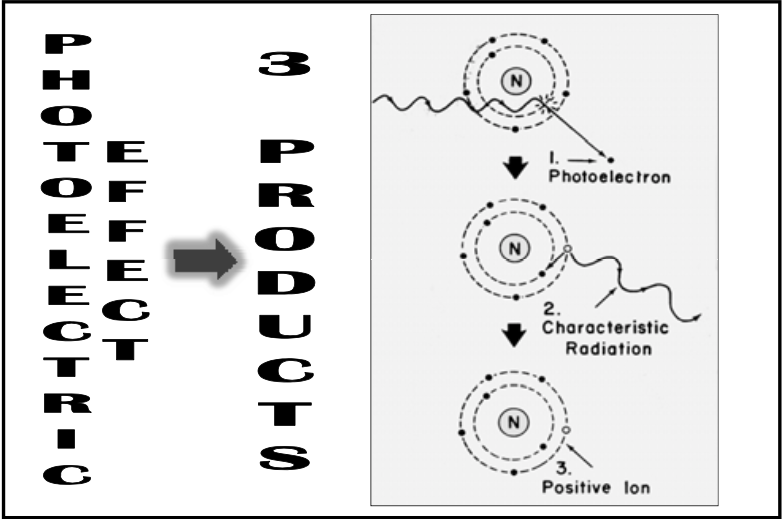
![<p>Photoelectric Reaction</p><ul><li><p>3 basic products:<br></p><ol><li><p><span><strong>[...]</strong></span></p></li><li><p><span><strong>[...]</strong></span></p></li><li><p><span><strong>[...]</strong></span> </p></li></ol></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/6dd61212-a45f-423e-9917-6def71192f0b.png)
Photoelectric Reaction
3 basic products:
[...]
[...]
[...]
Photoelectron
Characteristic radiation
Positive ion
![<p>Photoelectric Reaction</p><ul><li><p>Photon is absorbed<br></p><ul><li><p><span><strong>[describe the process.]</strong></span></p></li></ul></li><li><p>Atom resolves energy imbalance by <span>dropping an outer orbital electron</span> </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/fbfa35e7-cb47-4e98-9859-45fa4684daf0.png)
Photoelectric Reaction
Photon is absorbed
[describe the process.]
Atom resolves energy imbalance by dropping an outer orbital electron
incident photon interacts with an inner orbital, K or L, electron with an energy higher than the electron’s binding energy, which causes the electron to be ejected & the photon to be absorbed
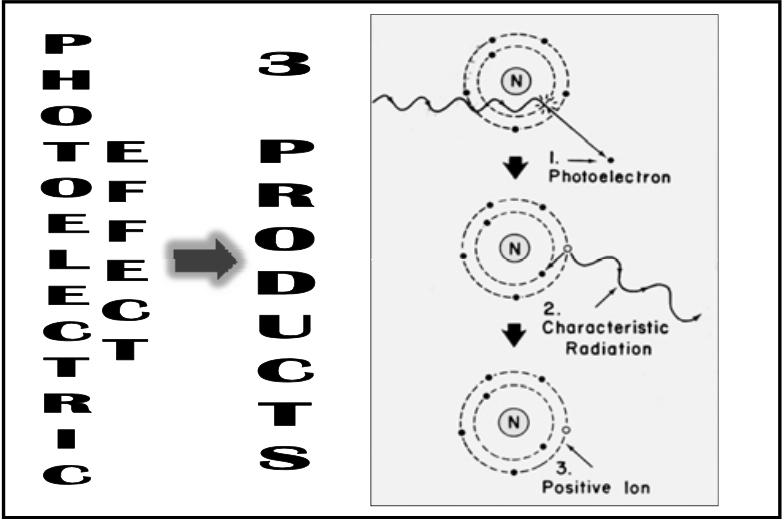
![<p>Photoelectric Reaction</p><ul><li><p>Photon is absorbed<br></p><ul><li><p><span>incident photon interacts with an inner orbital, K or L, electron with an energy higher than the electron’s binding energy, which causes the electron to be ejected & the photon to be absorbed</span></p></li></ul></li><li><p>Atom resolves energy imbalance by <span><strong>[...]</strong></span> </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/50fffb16-0475-4edc-a03e-7a0c8ac2ba35.png)
Photoelectric Reaction
Photon is absorbed
incident photon interacts with an inner orbital, K or L, electron with an energy higher than the electron’s binding energy, which causes the electron to be ejected & the photon to be absorbed
Atom resolves energy imbalance by [...]
dropping an outer orbital electron
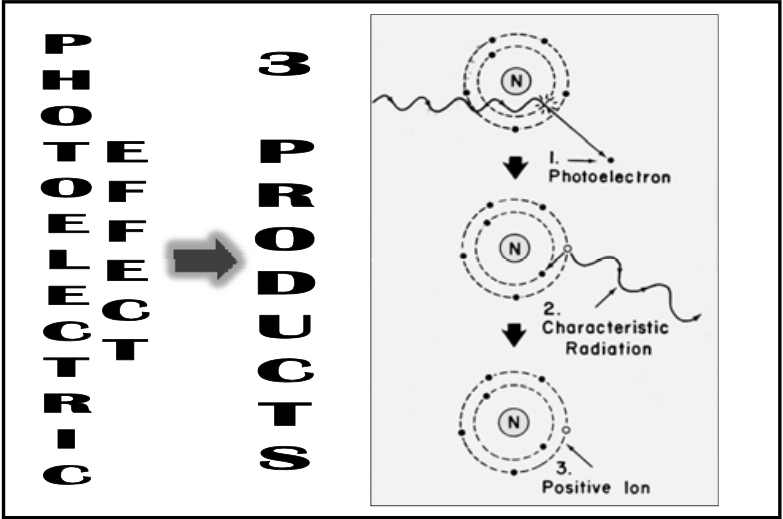
![<p>Photoelectric Reaction</p><ul><li><p>Similar to spike of characteristic spike except for some key differences:<br></p><ul><li><p>Atomic number is much <span><strong>[higher or lower]</strong></span> than Tungsten</p></li><li><p><span>Photon is coming in and knocking an electron out of its orbital instead of another electron coming in</span></p></li><li><p>Much <span><strong>[higher or lower]</strong></span> energy </p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/9bf4b8e9-f112-4066-b92b-6d7150fb0a7f.png)
Photoelectric Reaction
Similar to spike of characteristic spike except for some key differences:
Atomic number is much [higher or lower] than Tungsten
Photon is coming in and knocking an electron out of its orbital instead of another electron coming in
Much [higher or lower] energy
lower
lower
![<p>Photoelectric Reaction</p><ul><li><p>Similar to spike of characteristic spike except for some key differences:<br></p><ul><li><p>Atomic number is much <span>lower</span> than Tungsten</p></li><li><p><span><strong>[what knocks the electron out of its orbital in a photoelectric reaction]</strong></span></p></li><li><p>Much <span>lower</span> energy </p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/7a2bb3e4-1025-47e5-98fc-1421f5b31448.png)
Photoelectric Reaction
Similar to spike of characteristic spike except for some key differences:
Atomic number is much lower than Tungsten
[what knocks the electron out of its orbital in a photoelectric reaction]
Much lower energy
Photon is coming in and knocking an electron out of its orbital instead of another electron coming in
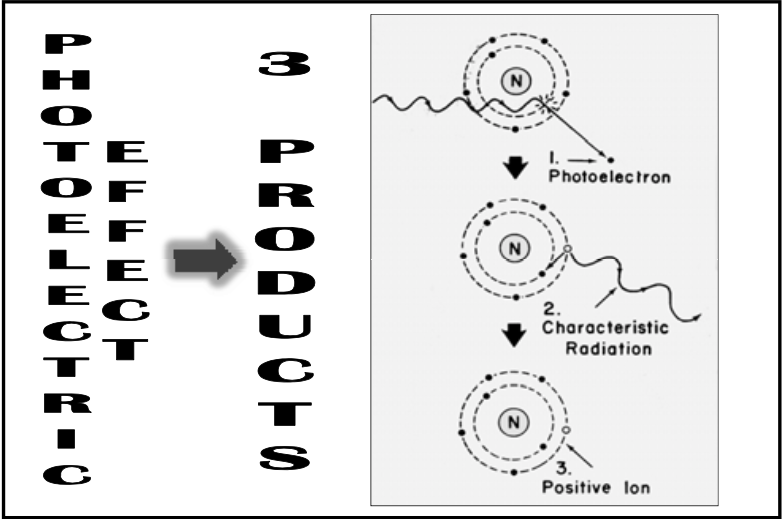
![<p>Photoelectric Reaction</p><ul><li><p>Yields <span><strong>[strong or weak]</strong></span> characteristic radiation in biologic system <br></p><ul><li><p><span>Much lower energy so it can’t travel for long before it is completely absorbed (if it’s a human, it won’t leave the body)</span> </p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f2f9ee3b-109b-49ac-93bd-afed9cd6e8be.png)
Photoelectric Reaction
Yields [strong or weak] characteristic radiation in biologic system
Much lower energy so it can’t travel for long before it is completely absorbed (if it’s a human, it won’t leave the body)
weak
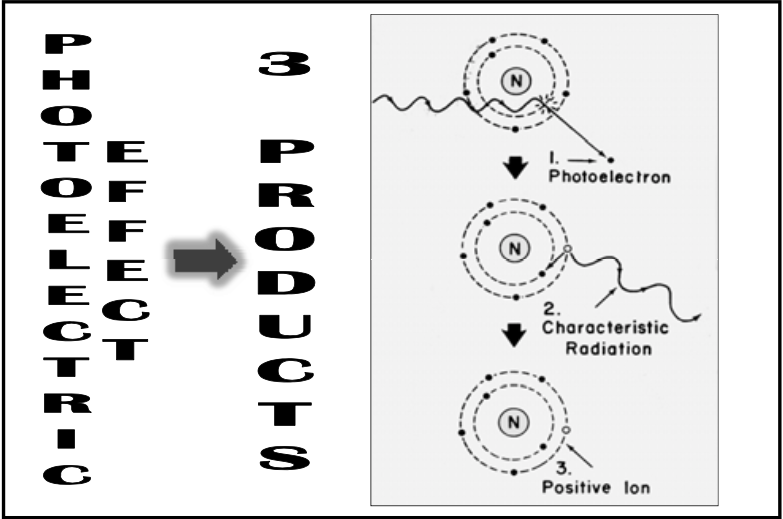
![<p>Photoelectric Reaction</p><ul><li><p>Yields <span>weak</span> characteristic radiation in biologic system <br></p><ul><li><p><span><strong>[what is the significance of this?]</strong></span> </p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/274a0265-0c33-4616-93d4-5f953abb396a.png)
Photoelectric Reaction
Yields weak characteristic radiation in biologic system
[what is the significance of this?]
Much lower energy so it can’t travel for long before it is completely absorbed (if it’s a human, it won’t leave the body)
![<p>Polyenergetic Attenuation</p><ul><li><p>When going through more obstacles, a polyenergetic beam <span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/c624324d-1db2-4533-9143-0296227aa03a.png)
Polyenergetic Attenuation
When going through more obstacles, a polyenergetic beam [...]
decreases in quantity and increases in quality
![<p><span>Polyenergetic beam becomes <strong>[...]</strong> after 3 to 4 HVL’s </span></p>](https://knowt-user-attachments.s3.amazonaws.com/4922f445-3353-4e33-b571-6bee198a2935.png)
Polyenergetic beam becomes [...] after 3 to 4 HVL’s
functionally monoenergetic
![<p>Radiation intensity varies inversely with the <strong>distance squared from the source</strong></p><ul><li><p>Due to <span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f4607684-5a4c-4816-a960-ea2e785d1307.png)
Radiation intensity varies inversely with the distance squared from the source
Due to [...]
the X-Ray beam divergence
shifting the peak indicates a change in [...]
changing the area under the curve indicates a change in [...]
quality
quantity
![<p>the tighter electrons are bound in orbit, photoelectric reaction is more likely</p><ul><li><p>Photoelectric reaction likelihood = <span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/4babac8a-4129-4373-97e8-60a7db4b9a38.png)
the tighter electrons are bound in orbit, photoelectric reaction is more likely
Photoelectric reaction likelihood = [...]
(atomic number)3
Higher atomic number = higher BE = tighter the electrons are bound
so higher the atomic number, the more likely a PE reaction would occur
![<p><span>the tighter electrons are bound in orbit, photoelectric reaction is <strong>[more or less]</strong> likely </span></p>](https://knowt-user-attachments.s3.amazonaws.com/1a8adda7-ef0f-468f-839f-b0af3b82694a.png)
the tighter electrons are bound in orbit, photoelectric reaction is [more or less] likely
more
P.E. = Z3
![<p>To get through a thicker layer, you can either increase kVp or increase mAs. What is safer?</p><ul><li><p><span><strong>[...]</strong></span> </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/c1b6211a-2e3a-4077-bc65-d54d71360020.png)
To get through a thicker layer, you can either increase kVp or increase mAs. What is safer?
[...]
kVp’s is actually saver
more radiation is deposited by low kVp
Two basic factors affecting quality of x-ray beam photons (shifts the curve)
[...]
[...]
Kilovoltage (kVp)
Filtration
But the only one that can be changed is the kVp because filtration is required by law
Vocabulary
[...]: photons pass through unaffected
Matter is NOT ionized
[...]: transfer energy to absorbing medium
Matter IS ionized
[...]: photons change energy and possibly lose energy
Matter MAY OR MAY NOT be ionized
Penetrate
Absorbed
Scattered
Vocabulary
Penetrate: photons pass through unaffected
[Is matter ionized?]
Absorbed: transfer energy to absorbing medium
[Is matter ionized?]
Scattered: photons change energy and possibly lose energy
[Is matter ionized?]
Matter is NOT ionized
Matter IS ionized
Matter MAY OR MAY NOT be ionized
![<p><span>What does the term "beam hardening" mean? </span></p><ul><li><p><span><strong>[...]</strong></span> </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/abaeafea-9757-4a19-bf56-172cc81fe3eb.png)
What does the term "beam hardening" mean?
[...]
increaseing effective beam energy through preferential loss of lower (softer) energy photons
![<p><span>What has the lowest HVL & what does that indicate? </span></p><ul><li><p><span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/7fe80b83-96f6-450f-9e49-0a56ae699e55.png)
What has the lowest HVL & what does that indicate?
[...]
Lead is the most efficient at cutting down x-ray beam so it is the best shield
A much thinner lead filter can do the same work as thicker cm. of muscle or bone
High-Value Layer: thickness of an aluminum absorber that is required to reduce beam intensity by 50%
![<p><span>What is the ONLY interaction between x-ray and matter that does not cause ionization? </span></p><ul><li><p><span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f021ab14-99c6-448a-851b-df19e27e0982.png)
What is the ONLY interaction between x-ray and matter that does not cause ionization?
[...]
Coherent Scatter
Low energy photons are simply absorbed & causes a vibration of the electron cloud before it goes back to the same energy
![<p><span>What is the relationship between beam intensity and kVp? </span></p><ul><li><p><span><strong>[...]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/4cd2305b-72c7-4313-877e-28e1e7a2e0f0.png)
What is the relationship between beam intensity and kVp?
[...]
Beam intensity is directly proportional to kVp2
Defines the quantitative effect
It is exponential!!!
Doubling the kVp increases beam intensity fourfold & vice versa
[...]
Measures the amount of radiation energy (E) absorbed per unit mass (M) of the absorbing medium
Medium must be specified
Absorbed Dose (D)
[...]: reduction of energy as energy passes through matter
Attenuation
![<p><span><strong>[...]</strong></span></p><ul><li><p>Typically <strong>low energy interactions</strong> in which radiation undergoes <span>a change in direction but no changes in wavelength (aka energy)</span> </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/e1f38257-ab36-4a83-a774-859ba1e98c09.png)
[...]
Typically low energy interactions in which radiation undergoes a change in direction but no changes in wavelength (aka energy)
Coherent Scattering
Low energy photons are simply absorbed & causes a vibration of the electron cloud before it goes back to the same energy
![<p><span>Coherent Scattering</span></p><ul><li><p>Typically <strong>low energy interactions</strong> in which radiation undergoes <span><strong>[...]</strong></span> </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/57df9bcf-fb98-4d59-9c46-949b24a19972.png)
Coherent Scattering
Typically low energy interactions in which radiation undergoes [...]
a change in direction but no changes in wavelength (aka energy)
Low energy photons are simply absorbed & causes a vibration of the electron cloud before it goes back to the same energy
![<p><span><strong>[...]</strong></span></p><ul><li><p>Description<br></p><ul><li><p>Photon interacts with outer orbital electron, imparting some energy to the outer orbital electron and ejects it from the orbit</p><ul><li><p>Ejected electron is called the <span>Compton electron</span>, which has an energy equal to the excess imparted by the photon</p></li></ul></li><li><p><span>Photon, as a result, is scattered with less energy due to the collision (less energy means larger wavelength in the end)</span></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/d1fef212-fad3-46c7-85fc-4a704f4f7e27.png)
[...]
Description
Photon interacts with outer orbital electron, imparting some energy to the outer orbital electron and ejects it from the orbit
Ejected electron is called the Compton electron, which has an energy equal to the excess imparted by the photon
Photon, as a result, is scattered with less energy due to the collision (less energy means larger wavelength in the end)
Compton Scatter
![<p><span>Compton Scatter</span></p><ul><li><p>Description<br></p><ul><li><p>Photon interacts with outer orbital electron, imparting some energy to the outer orbital electron and ejects it from the orbit</p><ul><li><p>Ejected electron is called the <span><strong>[...]</strong></span>, which has an energy equal to the excess imparted by the photon</p></li></ul></li><li><p><span>Photon, as a result, is scattered with less energy due to the collision (less energy means larger wavelength in the end)</span></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/611b3e94-79f2-4721-8777-786de89879bb.png)
Compton Scatter
Description
Photon interacts with outer orbital electron, imparting some energy to the outer orbital electron and ejects it from the orbit
Ejected electron is called the [...], which has an energy equal to the excess imparted by the photon
Photon, as a result, is scattered with less energy due to the collision (less energy means larger wavelength in the end)
Compton electron
![<p><span>Compton Scatter</span></p><ul><li><p>Description<br></p><ul><li><p>Photon interacts with outer orbital electron, imparting some energy to the outer orbital electron and ejects it from the orbit</p><ul><li><p>Ejected electron is called the <span>Compton electron</span>, which has an energy equal to the excess imparted by the photon</p></li></ul></li><li><p><span><strong>[what's the energy of the resultant photon?]</strong></span></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/10921f58-bb62-461d-ae23-3989186de3fb.png)
Compton Scatter
Description
Photon interacts with outer orbital electron, imparting some energy to the outer orbital electron and ejects it from the orbit
Ejected electron is called the Compton electron, which has an energy equal to the excess imparted by the photon
[what's the energy of the resultant photon?]
Photon, as a result, is scattered with less energy due to the collision (less energy means larger wavelength in the end)
[...]
Attempts to quantify biologic damage from deposition in tissues
Dose Equivalent (H)
![<p><span><strong>[...]</strong></span>: source related term used to express intensity of an X-Ray</p><ul><li><p>Source is basically the anode</p></li><li><p>Units<br></p><ul><li><p>SI System --> <span>Coulombs per kg (C/kg)</span></p></li><li><p>Non-SI System --> <span>Roentgens (R)</span> </p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/e1ab9c09-78ce-45de-a926-20809bc1e4dc.png)
[...]: source related term used to express intensity of an X-Ray
Source is basically the anode
Units
SI System --> Coulombs per kg (C/kg)
Non-SI System --> Roentgens (R)
Exposure
![<p><span>Exposure</span>: source related term used to express intensity of an X-Ray</p><ul><li><p>Source is basically the anode</p></li><li><p>Units<br></p><ul><li><p>SI System --> <span><strong>[...]</strong></span></p></li><li><p>Non-SI System --> <span><strong>[...]</strong></span> </p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/60adedf6-6b98-4321-b45a-85b0bfcc1ed2.png)
Exposure: source related term used to express intensity of an X-Ray
Source is basically the anode
Units
SI System --> [...]
Non-SI System --> [...]
Coulombs per kg (C/kg)
Roentgens (R)
[...]: thickness of an aluminum absorber that is required to reduce beam intensity by 50%
Half-Value Layer
![<p><span><strong>[...]</strong> = potential difference between cathode filament and anode target </span></p>](https://knowt-user-attachments.s3.amazonaws.com/004ce839-ad81-4f47-b69e-33cdcea56388.png)
[...] = potential difference between cathode filament and anode target
Kilovoltage (kVp)
![<p><span><strong>[...]</strong> has the highest effective anatomic number, indicating it is best option for a shield </span></p>](https://knowt-user-attachments.s3.amazonaws.com/b5a3319e-8c0b-4ab0-a4e3-8a2bb641f222.png)
[...] has the highest effective anatomic number, indicating it is best option for a shield
Lead
[...]
Energy absorbed by the medium per unit length of travel (keV per micrometer)
Proportional to (particle charge) 2
Linear Energy Transfer (LET)
Linear Energy Transfer (LET)
Energy absorbed by the medium per unit length of travel (keV per micrometer)
Proportional to [...]
(particle charge) 2
[...] and [...]
Ultra high energy interactions with matter that blows the nucleus apart
They do NOT occur in the diagnostic x-ray energy ranges
Might be helpful in radiation therapy
Pair Production and Photodisintegration
Pair Production and Photodisintegration
Ultra high energy interactions with matter that blows the nucleus apart
They do NOT occur in the diagnostic x-ray energy ranges
Might be helpful in [...]
radiation therapy
![<p><span><strong>[...]</strong></span></p><ul><li><p>Interaction with matter where x-ray is absorbed and not scattered </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/21baeaa0-686c-4652-a8c7-11926a059be2.png)
[...]
Interaction with matter where x-ray is absorbed and not scattered
Photoelectric Reaction
![<p><span><strong>[...]</strong></span> </p><ul><li><p>radiation that makes it through matter (could be the patient) to the other side </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/4ee34c52-82b9-41a8-b691-2299370a15a6.png)
[...]
radiation that makes it through matter (could be the patient) to the other side
Remnant Radiation