isomerism in transition metal complexes
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
what is an enantiomer?
isomers which are superimposable mirror images of each other
what is a chiral carbon?
C atom with 4 different attached groups
how does optical isomerism exist in transition metal complexes?
exists in octahedral complexes when the ion has 3 bidentate ligands bonded to it
these can ∴ form a pair of enantiomer
give an example of a transition metal complex displaying optical isomerism:
[Cu(en)3]2+
![<p>[Cu(en)<sub>3</sub>]<sup>2+</sup></p>](https://knowt-user-attachments.s3.amazonaws.com/d8fbc9f2-9512-4568-9ec7-8713bc8a957e.png)
what is cis-trans isomerism?
form of steroisomerism where identical groups can be positioned either adjacent (cis) or opposite (trans) each other
where can cis-trans isomerism be seen in transition metal complexes?
square planar complexes that have 2 pairs of ligands
octahedral complexes - 2 of one ligand, 4 of another
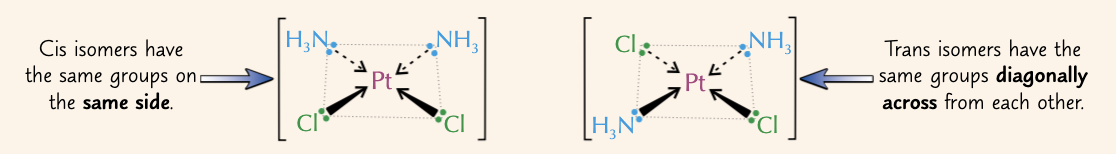
give a complex that displays cis-trans isomerism and name the two isomers:
PtCl2(NH3)2:
cis isomer = cisplatin
trans isomer = transplatin
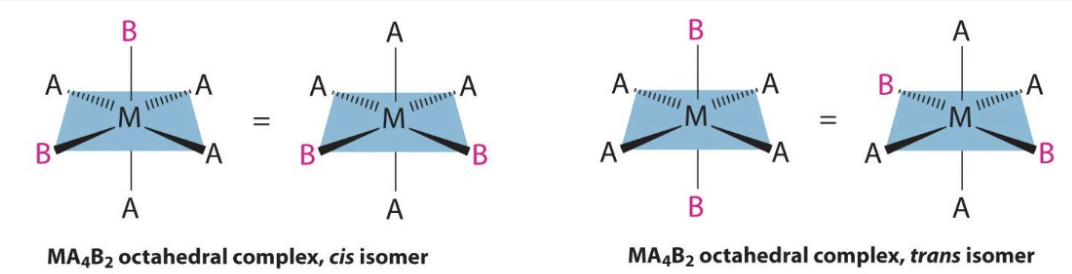
how do we distinguish between cis and trans isomers in octahedral complexes?
if the 2 ligands which are the same are opposite each other and so have an angle of 180o between them = trans
if the 2 ligands which are the same next to each other and so have an angle of 90o between them = cis