Materials
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
What are most drugs made up of?
The active ingredient (drug) and excipients.
What are excipients?
Inactive substances e.g., lubricants and diluents.
What do the properties of a solid formulation depend on?
The chemical composition and the solid structure of both drugs and excipients.
How can using a different solid form of the same drug
- Can alter the bioavailability of the drug.
- Can alter the stability of dosage forms.
- Can influence the way in which dosage forms can be processed and manufactured.
What are examples of solid forms of drugs?
Crystalline structures, polymorphs, salts etc.
What is bioavailablity?
the percentage of dose that enters systemic circulation.
Why is bioavailability important and what is it most affected by?
The drug must be in solution so it can cross the GI tract and is most affected by the solid form.
What is the rate limiting step of bioavailability/absorption?
The dissolution rate.
How can the solid form affect stability?
Different physical forms have different stabilities due to their structure and therefore can affect their chemical reactivity and may be more likely to convert to other forms.
How can the solid form affect the processing of drug?
Different physical forms may have different flows, compressions and water absorption and therefore affects their processing.
What is a molecule?
A distinct entity containing a number of atoms which are held together by covalent bonds.
What is a particle?
A distinct microscopic structure made up of millions of molecules.
What is a powder?
A visible mass of particles.
Describe crystalline materials.
Ordered with unit cell repeated in three dimensions and a have a specific melting point.
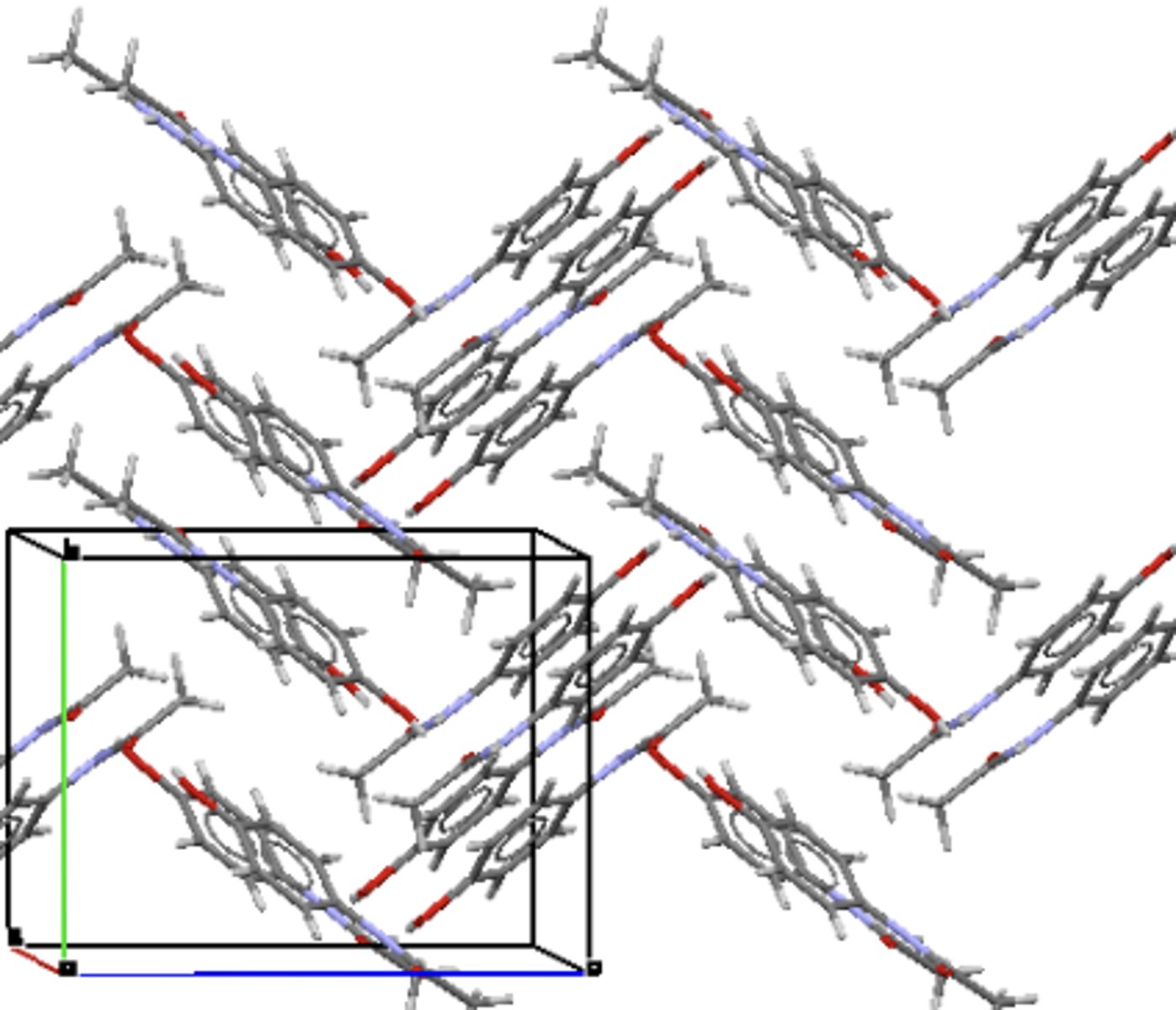
Describe amorphous materials.
- No long range order.
- Non-random local structure.
- Have a glass transition temperature (Tg).
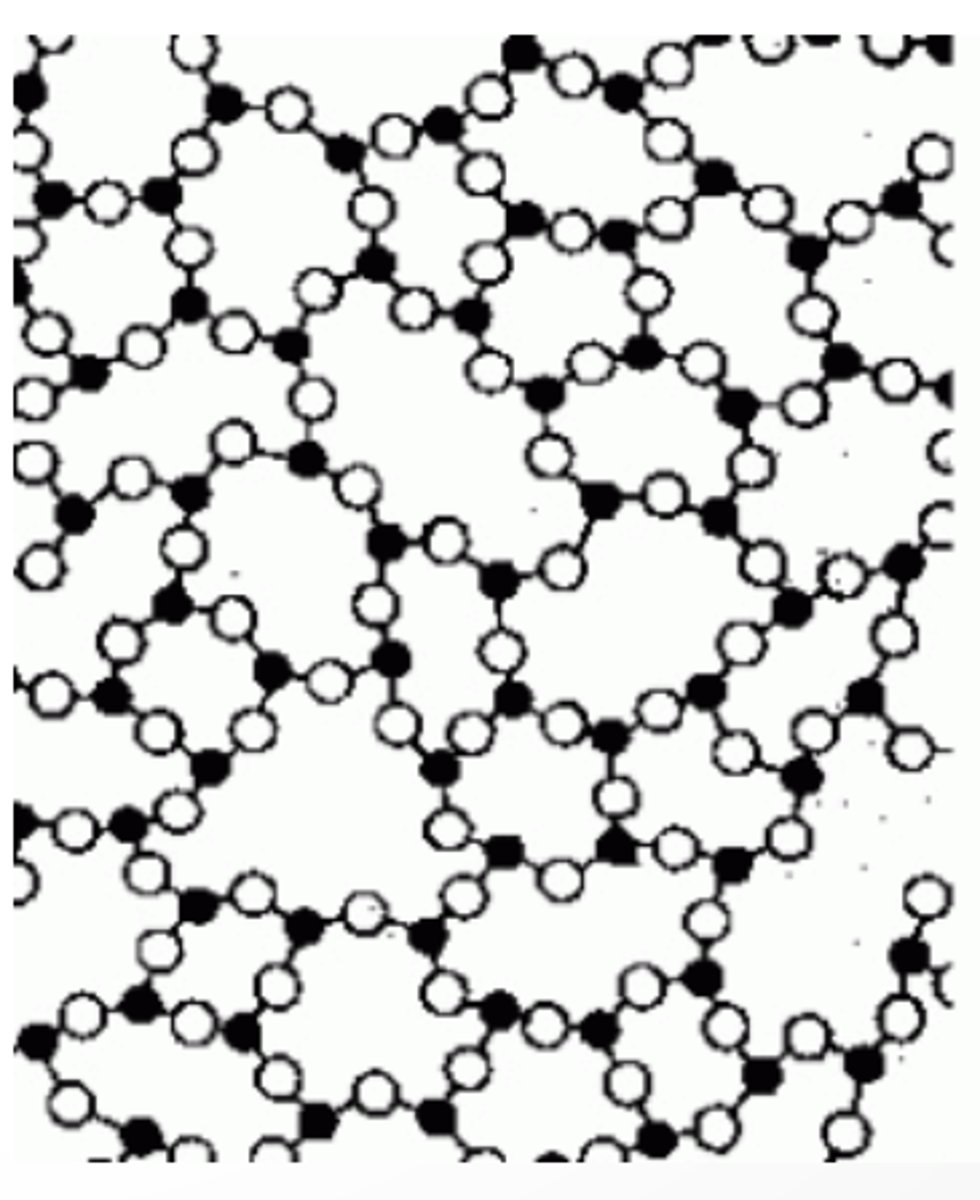
Describe crystal systems.
- Seven types of them
- Reflect the arrangement of molecules in the crystal.
- Often used to identify solid forms of drugs.
What are the seven types of crystal systems?
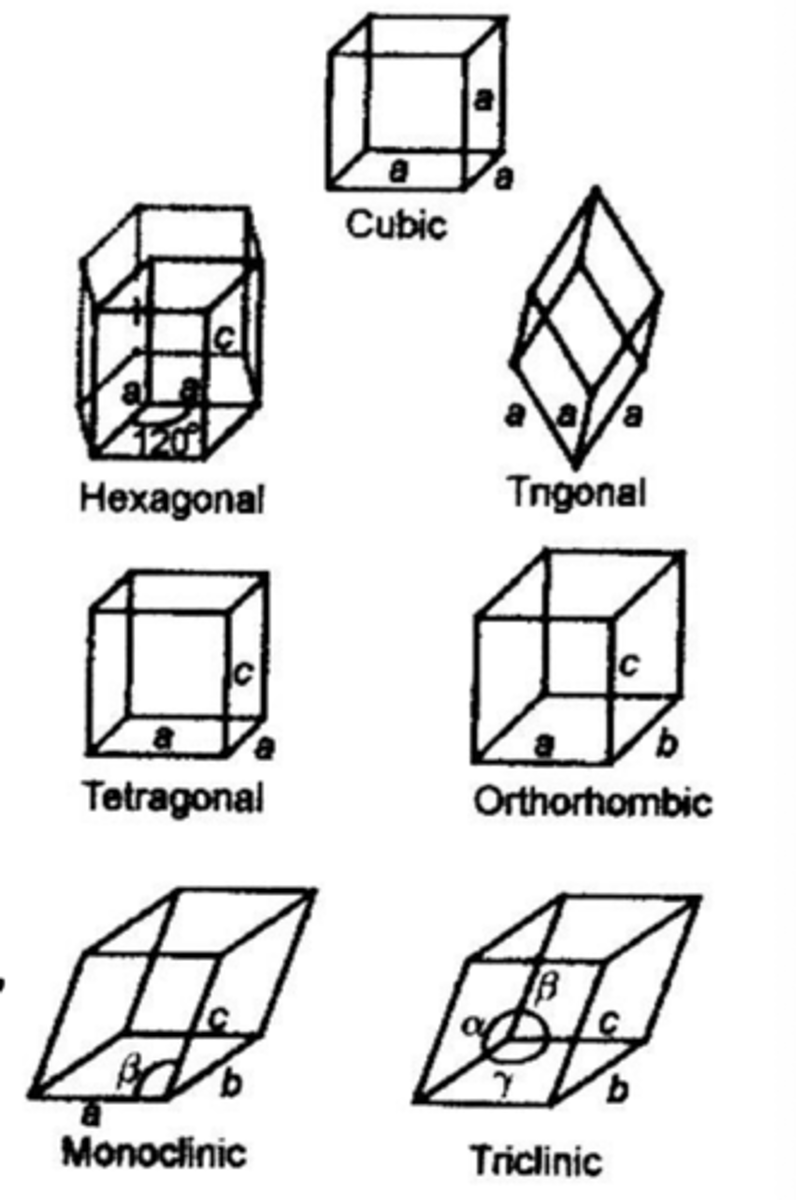
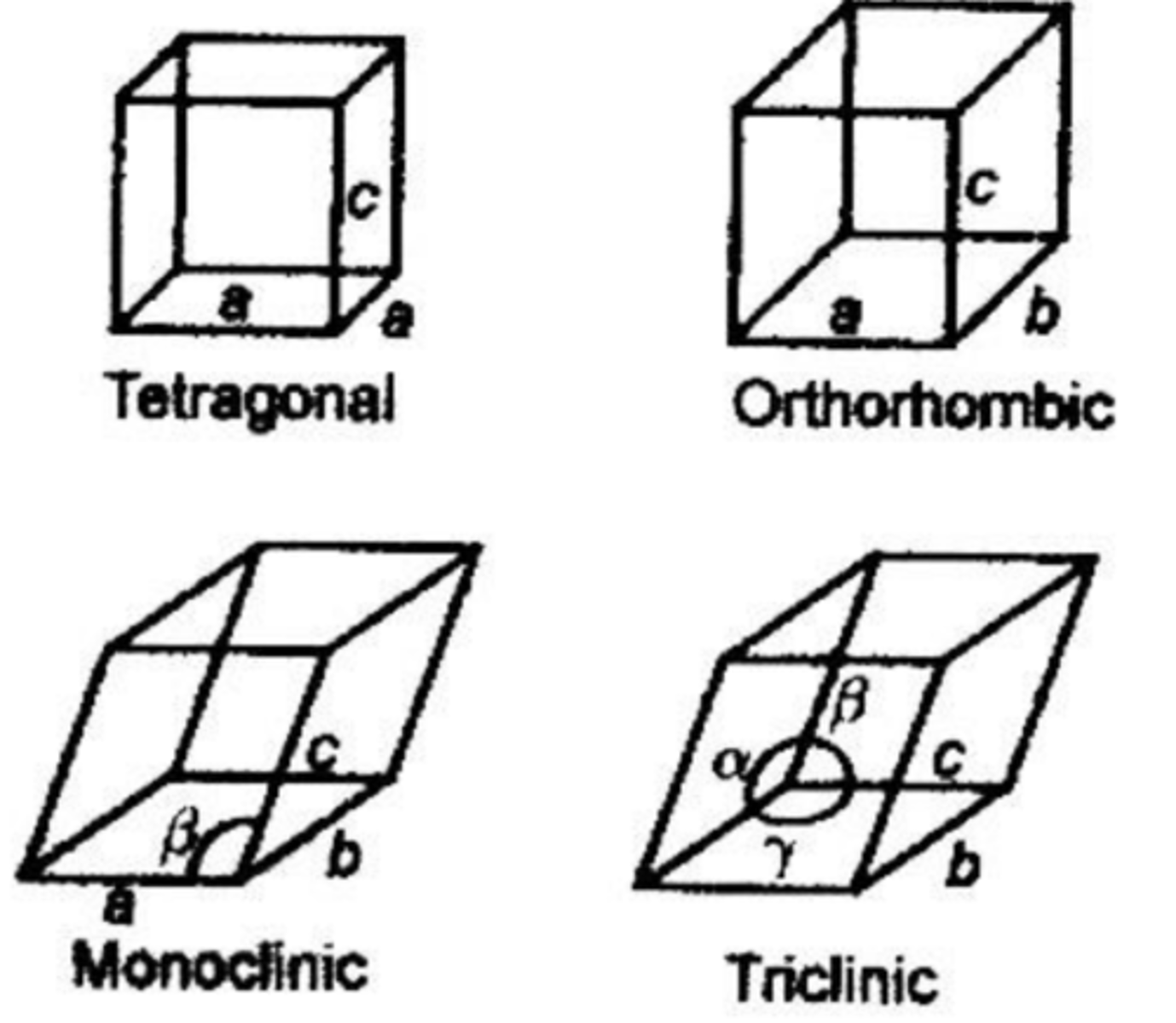
What crystal systems do drugs usually take up?
Most drug crystals are triclinic, monoclinic or orthorhombic.
What are polymorphs?
Minerals with the same composition but different crystalline structures.
Do polymorphs differ in physical properties?
Yes, they may have different melting points and solubilities however give the same solution/melt.
What form of polymorphs are stable at a given temperature?
The form with the lowest free energy (G) whilst the others are metastable.
What is metastability?
A partially stable state a system adopts in between stable states of organisation
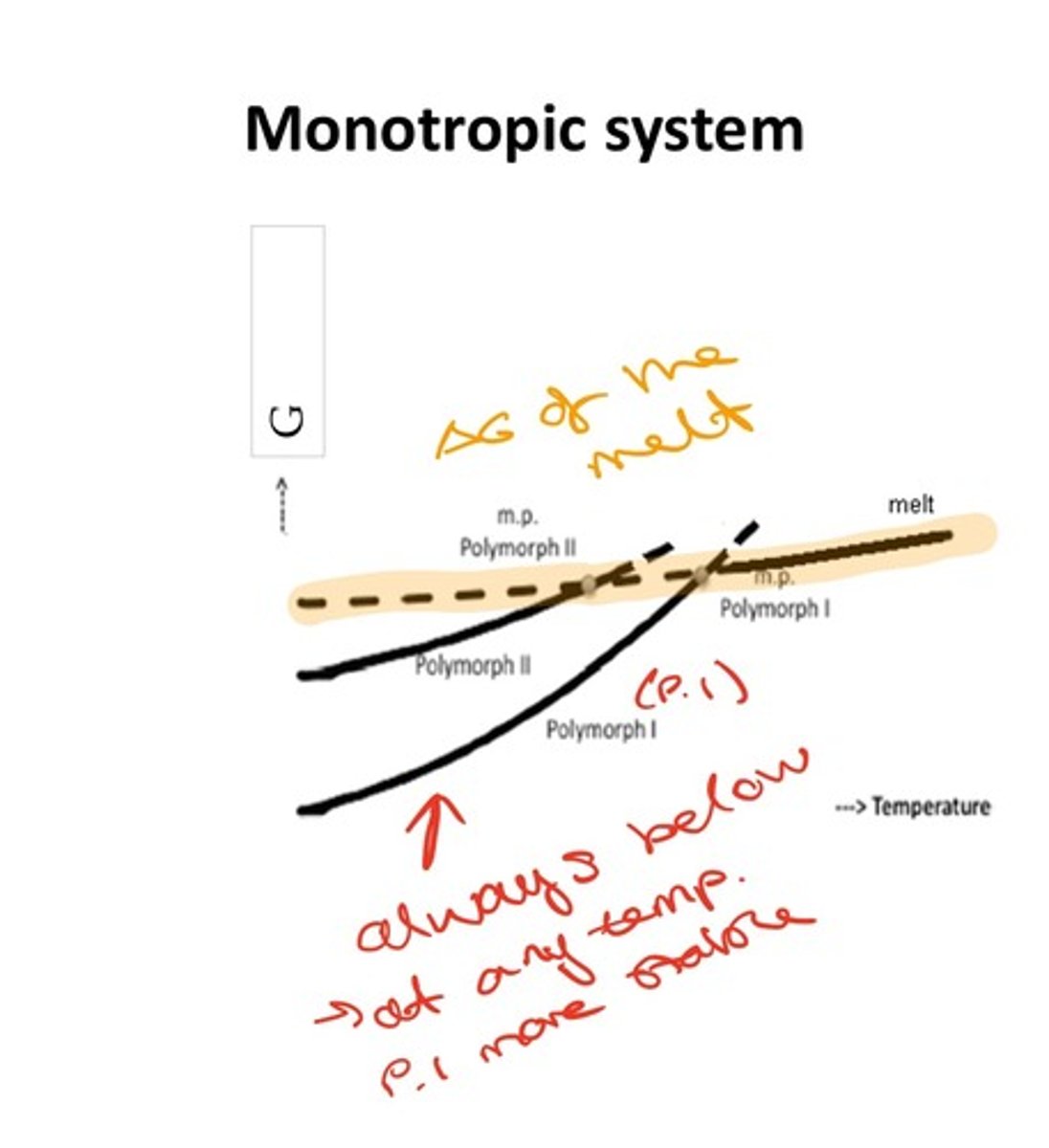
What is a monotropic relationship?
The same is stable irrespective of temperature.
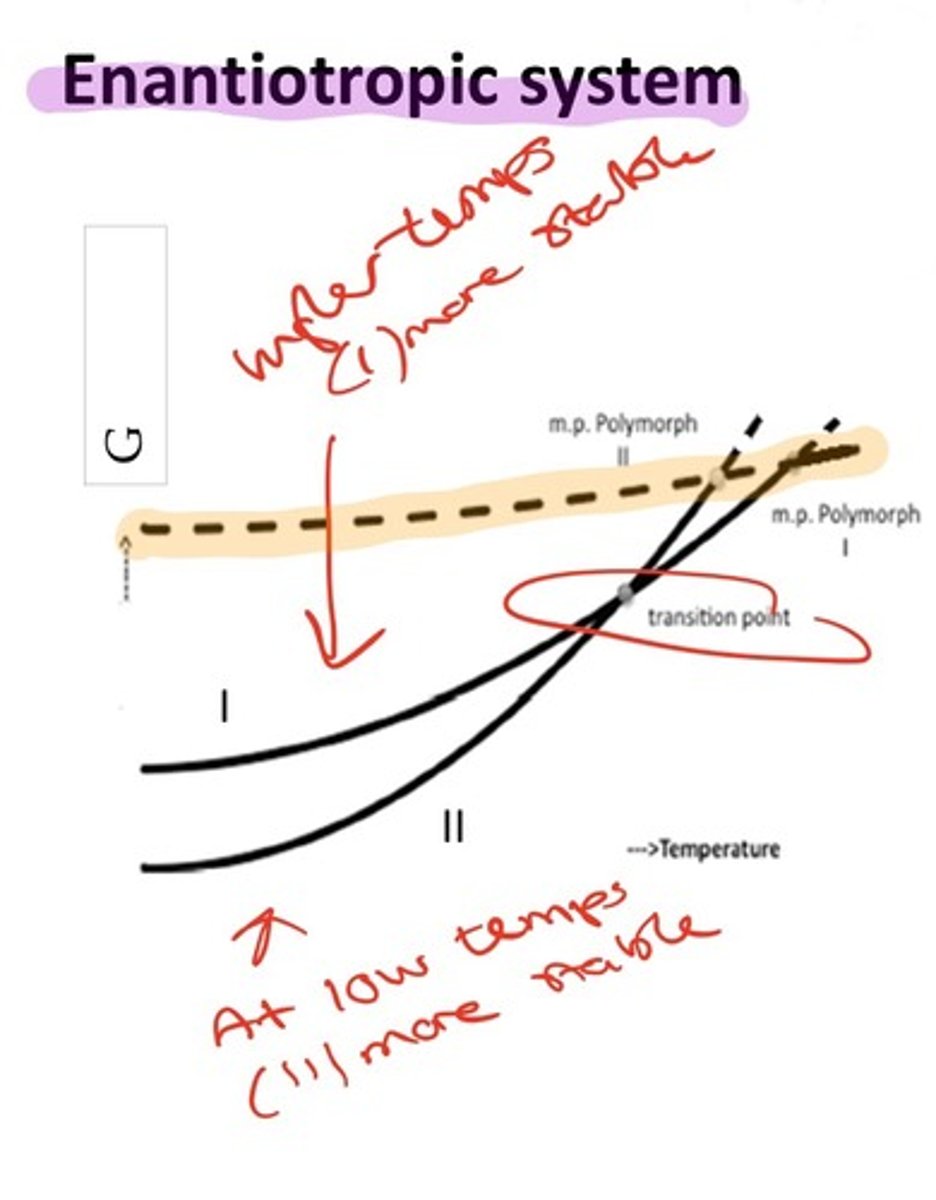
What is an enantiotropic relationship?
Either of two forms may be stable depending on the temperature e.g., below a critical temp.
Why is it difficult to tell whether you have a stable polymorph?
The metastable form may take a very long time to transform to the stable form.
How do crystals form?
Occurs in consecutive steps of:
- Supersaturation of solution.
- Nucleation
Describe supersaturation of solution during crystal formation.
Concentration of solution becomes higher than equilibrium solubility through cooling as drug solubility usually decreases with temperature.
Describe nucleation.
Very small particles form from the same materials as crystals (homegenous) or foreign materials e.g., dust (heterogenous). This requires energy until a critical size is reached.
Describe crystal growth.
Molecules diffuse to crystal surface and attach to the growing crystal.
What are the external shapes of the crystal?
Tabular, Platy, Prismatic, Acicular
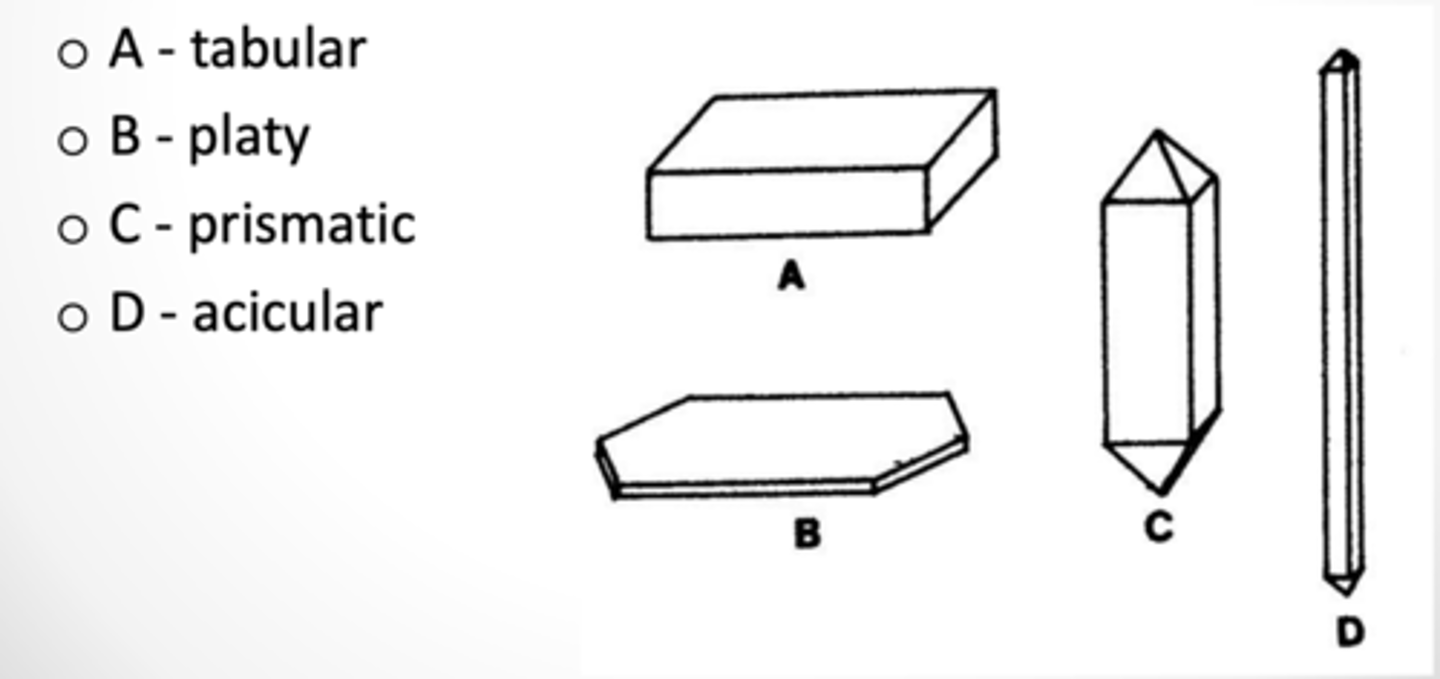
What is crystal habit?
Characteristic shape as it grows.
What can affect crystal habit?
Changes in crystal growth conditions such as the solvent and the cooling rate.
What effect does increasing the degree of supersaturation have on crystal growth?
Longer needles and thiner plates.
What is the habit of a crystal?
It's external shape.
What is the polymorph of a crystal?
The internal arrangement of the molecules in the crystal.
How are amorphous materials formed?
- Rapid cooling from the melt in order to prevent crystallisation.
- Fast precipitation from certain solvent systems e.g., antisolvent addition.
- High energy milling (disrupts crystals).
- Freeze drying.
Describe the dissolution of amorphous materials.
- Dissolves more easily than crystals as it is less stable.
- Produces a supersaturated solution which may crystallise.
What are issues with amorphous systems?
- All will recrystallise eventually.
- We do not understand systems as well as we should.
What is a solvate?
a crystal structure including a solvent molecule.
What is a hydrate?
a compound that contains water of crystallisation.
How are solvates formed?
Prepared by crystallisation with solvent and can form unexpectedly when drug is in contact with a liquid.
What are hydrate properties?
Hydrates may have different melting points, solubilities and dissolution rates compared to the anhydrous form
Describe the differences in solubilities of hydrates.
- Hydrates usually have lower solubilities and dissolution rates than anhydrous forms.
- However some hydrates have a higher solubility as water molecules can disrupt the crystal lattice.
- Non-aqueous solvates tend to have higher solubility than the anhydrous form.
Describe the difference between anhydrous dissolution and hydrate dissolution.
Anhydrous dissolution requrires breaking up of solid into molecules (endothermic) and interaction with solvent (exothermic). Whereas hydrate dissolution does not require interaction with water as it has already taken place therefore dissolution provides a lower energy gain as no exothermic process.
What can multi-component solid forms contain?
- The drug
- Another molecule/ion
- Solvates
- Salts
- Other solids called Co-crystals.