Microtubule Poisons
1/29
Earn XP
Description and Tags
VincaAlkaloid and Taxanes
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
What are microtubules and what is there cellular function?
Components (filament type) of the cytoskeleton. The cytoskeleton provides cellular shape and structure.
What are the three types of filaments in the cytoskeleton?
Actin Filaments
Tracks for myosin motors
Generate contractile forces, both in muscle and non-muscle cells.
Intermediate Filaments
Organise the 3-D structure of the cell for example, by anchoring organelles
Microtubules
Tracks for kinesins and dyneins
Microtubule Cellular Functions
Maintain the structure and shape of cells by providing structural support (cytoskeleton)
Provide a platform for Intracellular transport
Members of the two motor protein families, kinesins and dyneins move along microtubules in either the plus (cell periphery) and minus (cell center) ends respectively to unidirectionally transport a variety of cargoes such as vesicles, organelles and even chromosomes.
Involvement is meiosis and mitosis
Help in chromosome alignment and separation
Formation of spindle apparatus.
What are microtubules formed of?
Microtubules are formed of highly dynamic structures composed of tubulin protein dimers, which consist of alpha and beta tubulin subunits that polymerize to create hollow tubes.
They form stable heterodimers that assemble into linear protofilaments, ultimately forming the cylindrical structure of microtubules.
In each protofilament, the heterodimers are oriented with the beta tubulin monomer pointing towards the faster-growing plus end and the alpha tubulin monomer pointing towards the slower growing minus end.
What is microtubule growth inittiated by
y-tubulin
Capping proteins
usually on the - end
How does microbial growth occur?
by the addition of tubulins or dimers, a process driven by GTP hydrolysis called polymerisation
Microtubules can shrink or depolymerase.
This polymerisation and depolymerisation is responsible for the highly dynamic nature of microtubules in the cell.
Microtubules are particularly dynamic during mitosis where their turnover is about 20 minutes.
Cell cycle steps
G1: cell grows, duplication of organelles
S : DNA replication, chromosomes are duplicates
G2: cell grow, preparation for mitosis
Mitosis : cell divides
G1,S,G2: Interphase
What are the cell cycle checkpoints?
The asses DNA damage and if found, the cell stalls the cycle to repair the damage or if a repair can’t be made, targets the cell for destruction via the apoptotic pathway.
G1/S: everything is ready for DNA replication
G2/S: Everything is ready to enter mitosis
Mitotic Checkpoint: make sure chromosomes are properly aligned in the metaphase plate
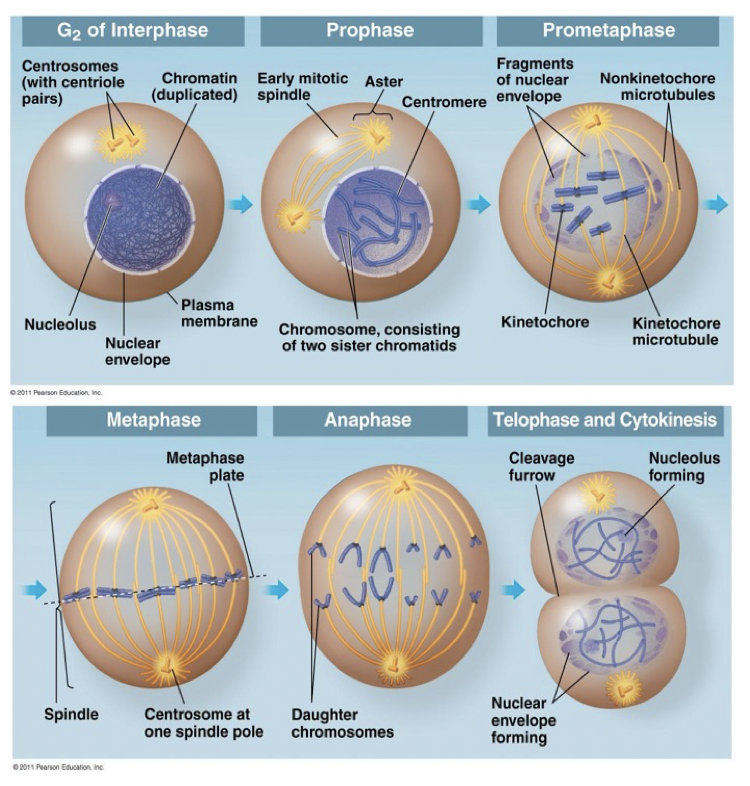
Microtubules Function in Mitosis
Prophase: duplicated centrosomes start to separate
Prometaphase: bipolar spindle starts to form. Chromosomes are captured by kinetochore MTs
Metaphase: Chromosomes are aligned at metaphase plate
Anaphase: sister chromatids start to separate, by moving to opposite spindle poles
Telophase & cytokinesis: genetic information is equally distributed between two daughter cells, which will physically separate at the end of process
What are two medicines known as Microtubule Poisons ?
Plant Vinca Alkaloid: Vincristine/Vinblastine
Taxanes: Paclitaxel
How do Vinca Alkaloid inhibitors work?
They depolymerase microtubules, and hence act as disruptors of MT assembly
Where are Vinca Alkaloids derived from
They are derived from the periwinkle plant Vinca rosea.
Natural products and semisynthetic derivatives
What is the difference between Vinblastine and Vincristine?
They are highly very structurally similar, they only vary in one position.
In this position, Vincristine has a formyl group substitute (R= CHO), while Vinblastine has a methyl group (R= CH3)
Mechanism of Action
They reversibly bind to:
Free alpha-beta-tubulin heterodimers
The ends of microtubules, where growth normally occurs (+ve end)
Therefore, Vinca Alkaloids disrupt the balance of the constant MT polyemrisation and depolymerisation by:
Blocking microtubule assembly (they stop the tubulin from building into microtubules)
Creating something called a “kinetic cap”, which stops any further growth
This therefore causes:
Dissolution of MTs: Microtubules break down
The mitotic spindle (needed to divide chromosomes during cell division) is destroyed
Mitotic arrest: Cells get stuck in mitosis (the division phase) and can’t continue
This leads to subsequent cell death
Uses of Vincristine
Leukaemia
Hodgkins and Non-Hodgkins lymphoma
Small cell lung cancer
Combination therapy: Multiple myeloma
Vinca Alkaloid Toxicity
Main Toxicity- Peripheral Neuropathy
Damage or disease affecting nerves
Pain and loss of deep tendon reflexes
Motor dysfunction, ataxia, and paralysis
Back, bone, and limb pains.
Neutropenia (DLT)
Where is Taxols dervied from?
Bark of Pacific Yew Tree
Taxol Mechanism of action
Stabilises microtubules (MTs) and protects them for disassembly or depolymerisation
This disrupts the normal dynamic balance of MT assembly/disassembly needed for mitosis.
MTs become abnormally bundled, and cells can’t divide properly.
Does taxol exhibits concentration-dependent effects?
Low doses: slow cell division
High doses: major structural abnormalities, mitotic arrest
What are the differences observed between effects of Taxals in cell culture and tumour?
o Tumours: can lead to multipolar spindles, abnormal mitoses, and eventually apoptosis (cell death)
In cell culture: mitotic arrest (cells freeze during division) and lagging chromsomes
Uses of Taxels
Lung, ovarian, breast, head and neck,
bladder, prostate and advanced forms of Kaposi’s sarcoma
Toxicity of Taxol
Neutropenia (Principal)
neurotoxicity
nausea & vomiting,
alopecia
myalgia (muscle pain),
hypersensitivity reactions
asthenia (weakness)
Anaphylactic Shock
Taxol Structure
Taxol (Paclitaxel) has a complex structure with 11 chiral centres, which contributes to its lipophilic (fat-soluble)nature.
However, due to this lipophilicity, Taxol is not naturally soluble in water, and thus requires a formulation to be administered intravenously.
How is Taxol formulated to be soluble for IV administration?
1. Cremophor EL
A polyethoxylated castor oil
Non-ionic surfactant, used to solubilise the drug and make it water miscible and bioavailable
Ethanol is also included in the formulation to help dissolve Taxol.
What Side effects is Cremophor EL resposnible for?
Anaphylactic shock (allergic reaction): This is a rare but serious side effect.
Phase I reactions: Up to 30% of patients may experience this, which includes hypersensitivity reactions, such as skin rash or difficulty breathing.
Why do the S/E happen?
Cremophor EL can induce histamine release and other allergic responses, leading to the risk of anaphylactic reactions.
The ethanol in the formulation may also contribute to some side effects, like flushing or discomfort at the injection site.
What is Abraxane
It is an Albumin Bound Taxol
Uses of Abraxane
It is used to treat Advanced breast cancer, non-small cell lung cancer, and advanced pancreatic cancer ((not justified anymore due to its limited benefits compared to current treatments).
Positives of Abraxne
1- Albumin Nanoparticle: In Abraxane, paclitaxel is bound to albumin (a naturally occurring protein in the body), forming nanoparticles. This binding helps paclitaxel be delivered more efficiently to tumor cells.
2- No Solvent Needed: Unlike Taxol, which requires Cremophor EL (a solvent that can cause side effects like anaphylaxis), Abraxane does not need a solvent for paclitaxel to be delivered. This reduces the risk of severe allergic reactions like anaphylactic shock that can occur with Taxol.
3- Abraxane allows for a much shorter infusion time—around 30 minutes—compared to 3 hours required for the traditional Taxol infusion.
This makes it more convenient for patients and reduces the time spent in treatment.
Why is a shorter infusion time in Abraxane?
Abraxane is already in a nanoparticle form, which allows it to be more easily transported through the bloodstream and delivered directly to the tumor site. The albumin molecules help paclitaxel to cross cell membranes more efficiently, reducing the need for a slow infusion to prevent adverse effects.