module 5 - physical chemistry & transition elements
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
techniques for measuring rate of reaction
mass loss
gas production
colorimetry
units of rate of reaction are typically
mol dm^-3 s^-1
calculating rate of reaction
change in concentration/ change in time
what is a colorimeter or spectrometer
measures the light intensity of light passing through a sample, intensity of light is measured every few seconds and data is plotted, light intensity is related to the concentration
measuring the rate of reaction using changes in mass
when gas is produced in a reaction it escapes from the reaction vessel so the mass of the vessel decreases, can be measured using balance, the cotton wool in the neck of the flask allows gas to escape
one limitation of measuring loss in mass
gas must be sufficiently dense or the change in mass is too small to measure on a 2 or 3 decimal balance
measuring rate of reaction using changes in volume of gas
gas collection can involve collecting gas through water by displacement using a burette or inverted measuring cylinder, this method can only work if gas has a low water solubility
collision theory
for a reaction to occur:
particles must collide with enough kinetic energy
rate of chemical reaction depends on how often successful collisions happen
what is collision theory affected by
how often particles collide
the energy each particle has when they collide
the minimum energy needed for reaction to occur: activation energy
collision geometry - orientation or angle when particles collide
what can collision frequency (how often particles collide) be altered by
change in total pressure
change in concentration of reactants
change in temperature
change in surface area
what affects rate of reaction
concentration
pressure
temperature
surface area
catalyst
how does pressure affect rate of reaction
the same number of particles occupy a smaller volume, resulting in increased collision frequency
how do catalysts impact rate of reaction
they lower activation energy so more particles will have sufficient energy to react
energy profile of exothermic reaction
energy of reactants are higher than products, change in products - reactants is less than 0
energy profile of endothermic reaction
products have higher energy than reactants and change in products - reactants is greater than 0, (positive 🔺H)
in a maxwell boltzmann graph, why do the particles never show having 0 energy ?
there will always be a small amount of particles that have very high energy
how does a catalyst impact the rate of reaction
it provides an alternative pathway for a reaction to occur, lowering the activation energy
how does raising the volume of a reactant impact the rate of reaction
it lowers the rate of reaction because the same amount of particles being in a larger space increases the distance between them, leading to lower frequency of successful collisions
what’s the relationship between the rate and concentration of reactant in a zero order reaction
the rate is independent of the concentration of the reactant → same amount of concentration decomposes over time
How can something which is zero order increase in conc but have no effect on rate?
For reaction : rate = k [C]1 [S]0 = k [C]1 x 1 = k [C]1
[S]omething can increase in concentration but have no effect on rate because the reaction between [C]1 & [S]0 has multiple steps like in organic mechanisms e.g. electrophilic subsitution etc.
This would mean there is an intermediate formed between Step 1 & Step 2 etc etc that is the one colliding etc and it is the rate of formation of this intermediate that effects the rate and not [S].
Why might there be multiple steps in a reaction where one substance is zero order?
It could be that the non-0 order chemical (C) undergoes a chemical change that has nothing to do with the 0 order one (S). It may even happen when C is in the beaker on its own! This can happen by the collision of a particle of C with a solvent molecule in which it is dissolved. This is a known process.
How could you explain & represent a reaction mechanism of rate = k [C]1 [S]0 = k [C]1 x 1 = k [C]1?
Now let’s say that C is being slowly converted into something new called π. Let's write an equation for this equilibrium process. Because this is an equilibrium process there will only be a limited amount of π at any time, especially if the equilibrium lies to the left:
STEP 1: C ⇌ π slow reaction
As soon as is π produced, it reacts with S in a very fast reaction to produce the product D. The equation is:
STEP 2: S + π ➔ D fast reaction
Overall, we add the equations together cancelling anything we can. We do this all the time in chemistry.
C ➔ π STEP 1(slow)
π + S ➔ D STEP 2 (fast)
C + S ➔ D Overall
If you wanted to include a solvent molecule, sol, in the process, you could write it as follows
C + sol ➔ π STEP 1(slow)
π + S ➔ D + sol STEP 2 (fast)
C + S ➔ D Overall (the solvent molecule was regenerated in step 2)
Why in multiple step reaction mechanisms can we not observe the reactive intermediate π?
This is because it doesn’t hang around. As soon as it is made, it is consumed very quickly by S.
If we looked hard enough, we might be able to capture some .
What more detailed, analgous explanation as to why something which is zero order increase in conc but have no effect on rate?
If the “reaction” is rate of dirty dishes produced by a bakery then our “reactants” will be the number of bakers and the number of customers.
If the number of customers increases, the rate of dirty dishes produced won’t increase.
This is because the “hidden intermediate” is number of pasteries baked since if the bakers can only make 2 pasteries only 2 dirty dishes will be produced even for a crowd of 1000.
This means only when number of bakers and so number of pasteries increases does rate of dirty dishes.
What can you assume for reaction mechanisms?
If any overall reaction has a chemical involved which is found to be zero order, you should assume that it must be a multi-step reaction, i.e. 2 or more steps.
At A Level, you should also assume that the first step in a multi-step reaction is the rate determining step.
How can you write a reaction mechanism from a rate equations/ orders of reactants e.g. NO2(g) + CO(g) ➔ NO(g) + CO2(g) with rate = k [NO2]2?
We have to make assumptions about the reaction and if at the end of the mechanism we get the reaction equation we have done it right
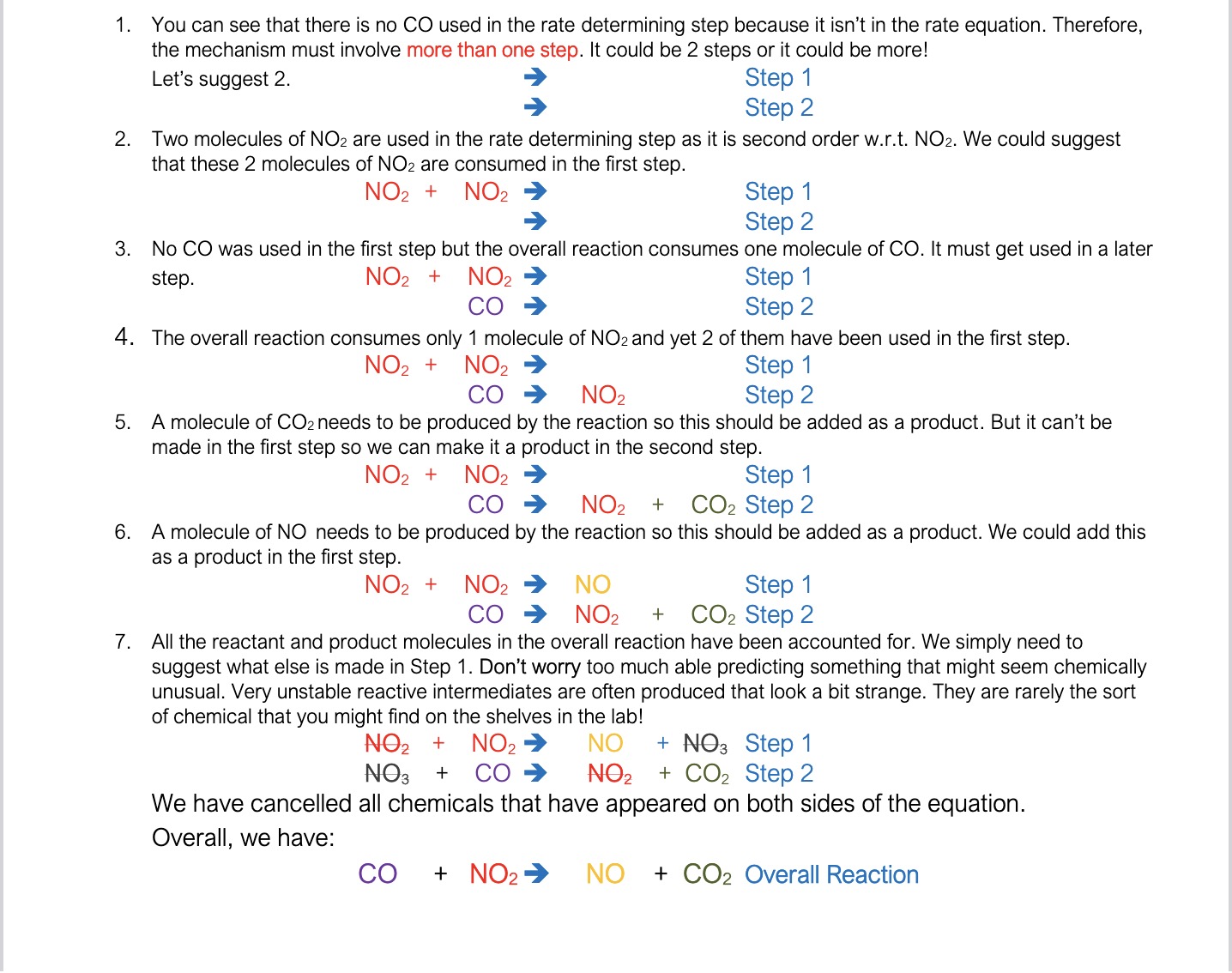
What are some “rules”/common assumptions to make when writing a reaction mechanism from orders of reactants etc?
If there is a chemical in the overall equation that is not in the rate equation, then it can’t be in the rate determining step. You should therefore assume that the reaction takes place in at least 2 steps.
If there is only molecule of a particular reactant in the overall reaction but it is second order w.r.t. to that reactant, then you know that you must use up 2 molecules in rate determining step but you must regenerate one molecule of the ‘reactant’ in a later step.
If there are 2 molecules of a particular reactant in the overall reaction but it is first order w.r.t. to that reactant, then you know that you must use up 1 molecule in rate determining step, but another reactant molecule must be consumed in a later step.
You can generally assume at A level that all orders are whole numbers. (Many reactions have non-integer orders but don’t worry about that at A level. I’m just being honest!)
You can generally assume that the rate determining step is the first step