Amino Acid Structure and Classification
1/38
Earn XP
Description and Tags
Amino Acids and Proteins CH
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
Amino acids
The essential building blocks of proteins.
Each amino acid contains what groups?
An amino group (NH2), a carboxyl group (-COOH), and a side chain (R group).
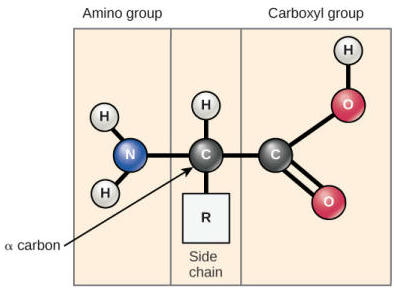
What does an amino acid structure look like?
Amino acids have a central carbon atom attached to an amino group, carboxyl group, hydrogen atom, and a variable R group. The R group determines the specific properties of each amino acid.
Amino acids are classified based on:
Hydrophilicity, charge, and aromaticity.
Alpha carbon
The central carbon atom in an amino acid to which the amino group, carboxyl group, hydrogen atom, and R group are attached.
Chiral
A property of the alpha carbon in amino acids, meaning it has four different groups attached unless R = H (as in glycine).
Nonpolar, Nonaromatic (Hydrophobic) Amino Acids Definition.
Amino acids with hydrophobic side chains that stabilize protein structures by avoiding water.
Nonpolar, Nonaromatic Amino Acids Types.
Includes Glycine (Gly), Alanine (Ala), Valine (Val), Leucine (Leu), Isoleucine (lle), Methionine (Met), and Proline (Pro).
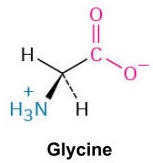
Glycine (Gly, G)
The simplest amino acid with a hydrogen atom as its side chain, providing flexibility to protein structures. It can be found inside and outside of proteins.
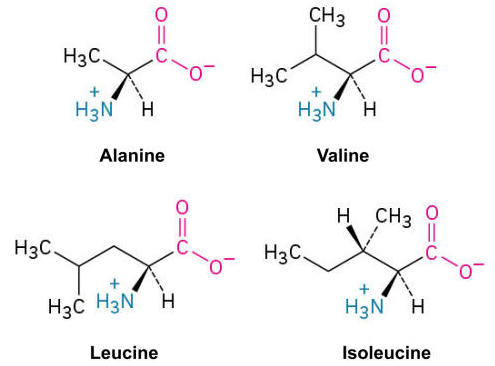
Alanine (Ala, A), Valine (Val, V), Leucine (Leu, L), and Isoleucine (Ile, I):
These amino acids have alkyl side chains of varying lengths, contributing to the hydrophobic core of proteins.
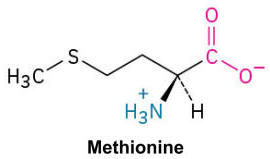
Methionine (Met, M)
An amino acid that contains a sulfur atom within a thioether group, often involved in initiating protein synthesis.
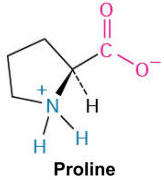
Proline (Pro, P)
This amino acid is unique due to its cyclic structure that includes the amino nitrogen, restricting flexibility; it's often found in turns and loops of proteins.
Nonpolar/hydrophobic aromatic amino acids (Aromatic Amino Acids)
These amino acids have aromatic rings in their side chains, which can absorb UV light, making them helpful in determining protein concentration via spectroscopy.
Aromatic amino acids Types.
Phenylalanine (Phe, F), Tryptophan (Trp, W)
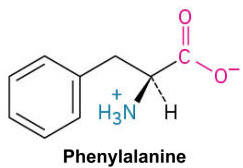
Phenylalanine (Phe, F)
A nonpolar amino acid with a benzyl side chain, contributing to the hydrophobic core of proteins.
Tryptophan (Trp, W)
This is the largest amino acid, with a complex double ring that includes a nitrogen atom, and it plays a vital role in protein-protein interactions.
Polar/ Hydrophilic Aromatic Amino Acid type.
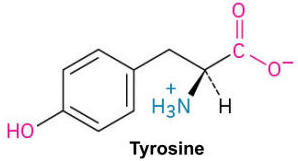
Tyrosine (Tyr, Y)
Tyrosine
A polar amino acid with a hydroxyl group attached to its benzyl ring, involved in signaling pathways.
Polar/Hydrophilic, uncharged amino acids (Polar, Uncharged amino acid)
These amino acids have side chains that can form hydrogen bonds with water, making them hydrophilic.
Polar, Uncharged amino acid types.
Include Serine (Ser, S), Threonine (Thr, T), Cysteine (Cys, C), Asparagine (Asn, N), and Glutamine (Gln,Q).
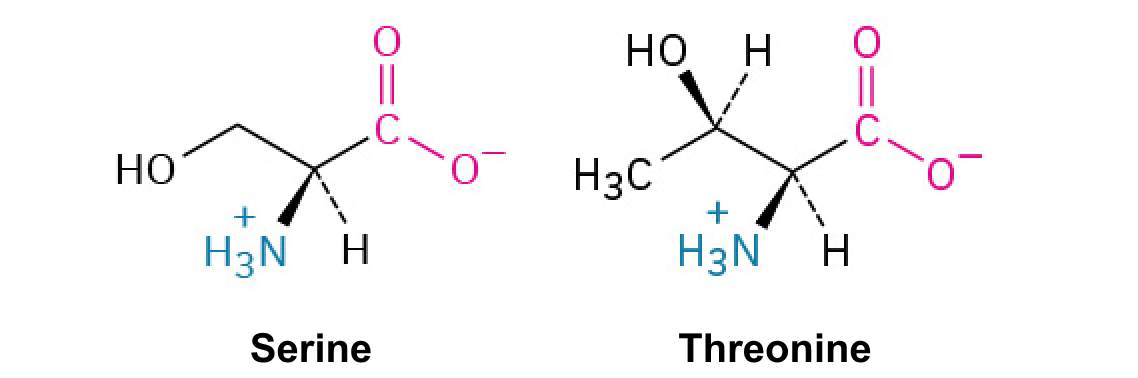
Serine (Ser, S) and Threonine (Thr, T)
These both contain hydroxyl groups, which are often involved in phosphorylation.
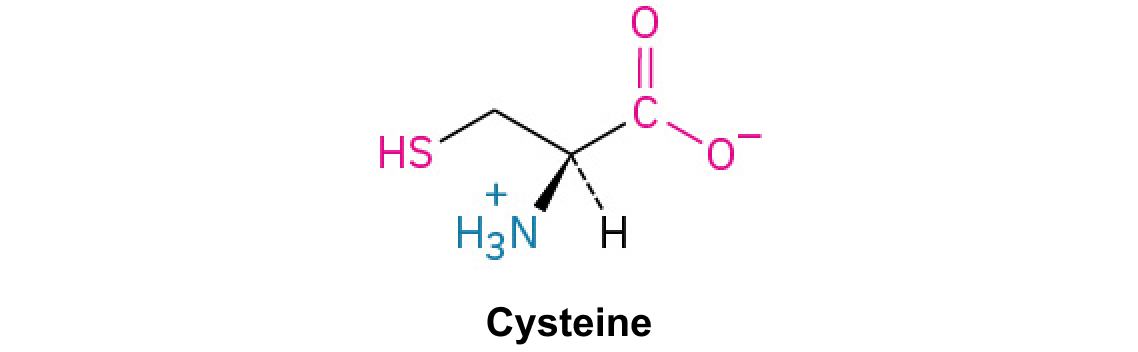
Cysteine
An amino acid with a thiol group that is reactive and can form disulfide bonds, crucial for protein stability.
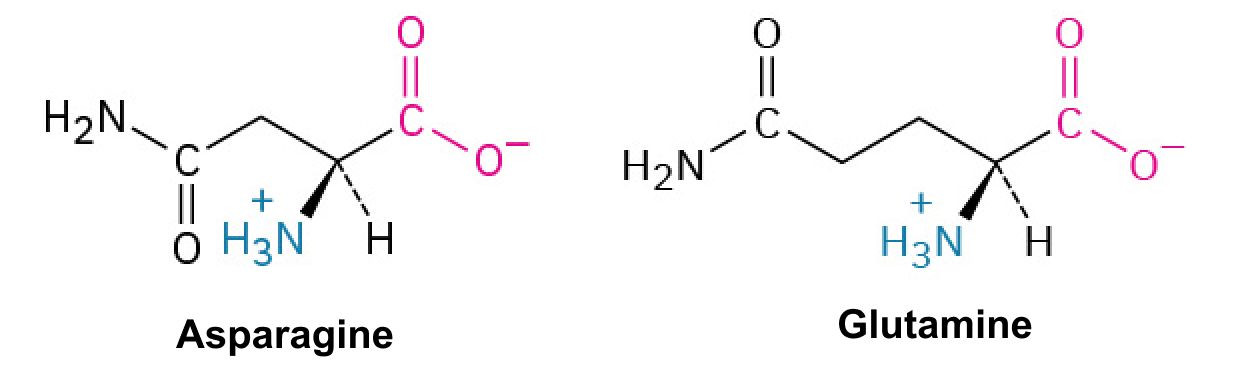
Asparagine (Asn, N) and Glutamine (Gln, Q)
These contain amide groups that do not gain or lose protons, remaining neutral in charge and often involved in hydrogen bonding.
Charged Amino Acids (Polar/Hydrophilic, Charged amino acids)
These amino acids can be divided into acidic or basic based on their side chain charges. They are often important for enzymatic activity and for creating electrostatic interactions that help to stabilize protein structure.
Charged Amino Acids Types
Aspartic acid (Asp, D, -), Glutamic (Glu, E, -), Lysine (Lys, K, +), Arginine(Arg, R, +), Histidine (His, H, ±).
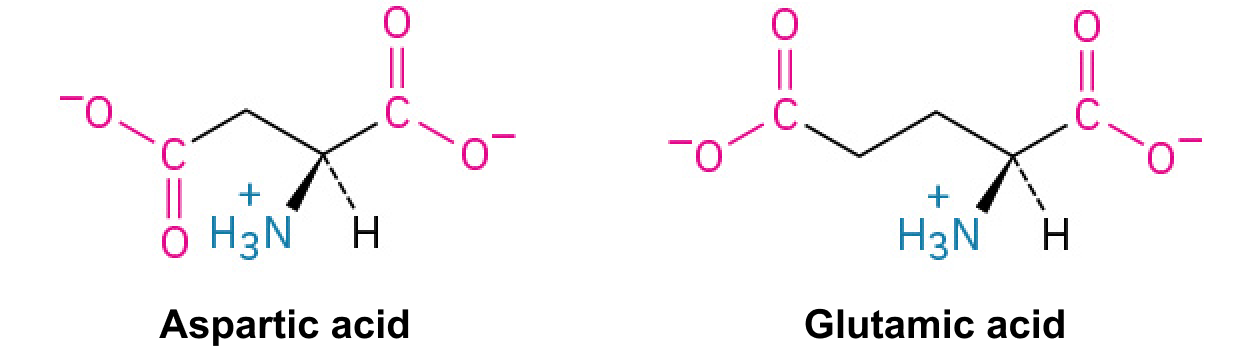
Aspartic Acid (Asp, D) and Glutamic Acid (Glu, E):
An acidic amino acid with a carboxyl group that loses protons, becoming negatively charged. They are often involved in active sites of enzymes.
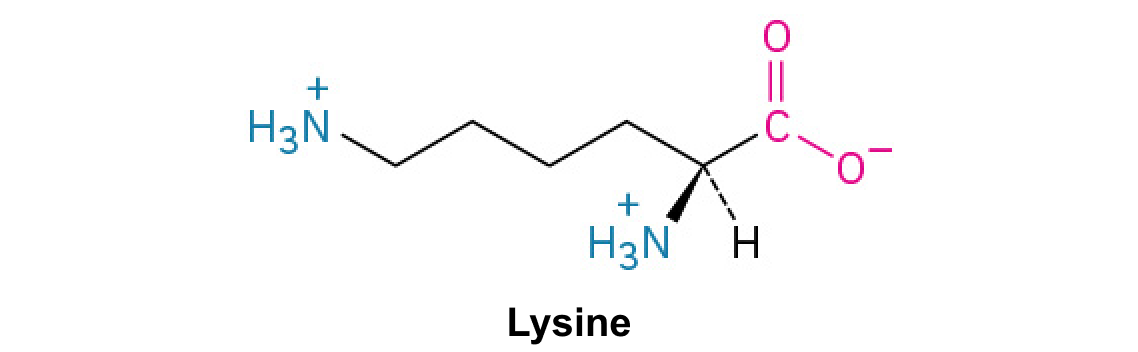
Lysine (Lys, K)
A basic amino acid with a terminal primary amino group, often binding to negatively charged molecules.
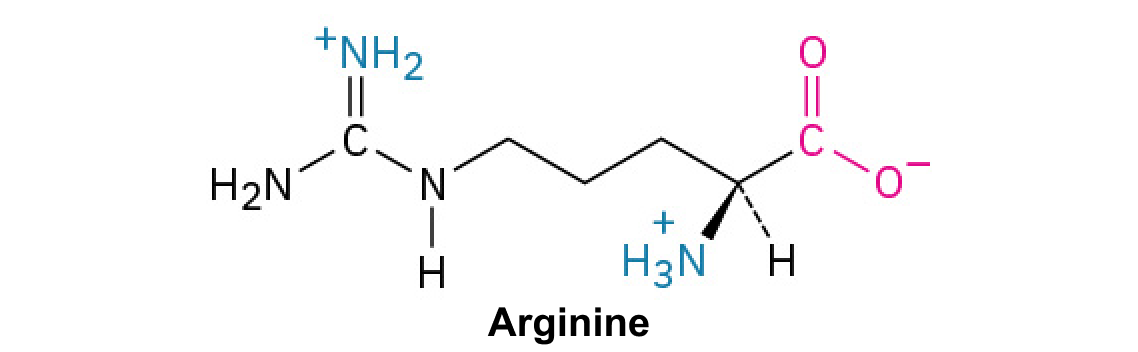
Arginine (Arg, R):
This amino acid contains a guanidinium group, making it highly basic and often involved in protein binding.
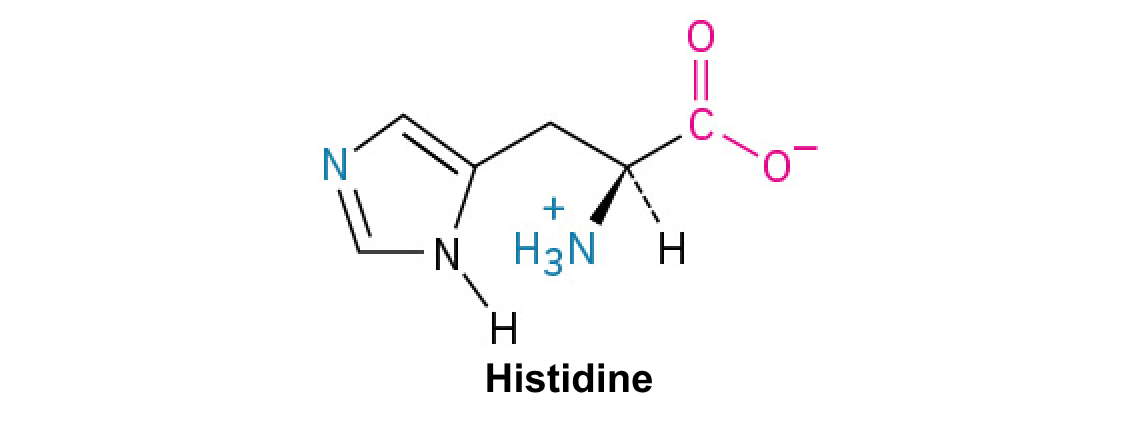
Histidine (His, H):
This amino acid contains an imidazole ring, which can shuttle protons, playing a crucial role in enzyme active sites and buffering.
Essential Amino Acids
Amino acids that cannot be synthesized by the human body and must be obtained from the diet.
Essential Amino Acids Types:
These include histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
Non-Essential Amino Acids.
Can be synthesized by the body.
Non-Essential Amino Acids Types:
These include alanine, asparagine, aspartic acid, glutamic acid, serine, arginine, cysteine, glutamine, glycine, proline, and tyrosine.
Post-Translational Modification.
Amino acids in proteins can undergo various post-translational modifications that affect protein function, localization, and interactions.
Post-Translational Modification Include:
Phosphorylation, Glycosylation, Acetylation, Methylation, and Ubiquitination.
Phosphorylation
The addition of phosphate groups to amino acids (usually to serine, threonine, or tyrosine), playing a critical role in signaling pathways.
Glycosylation
It is the attachment of carbohydrate groups, affecting protein folding, stability, and cell recognition.
Acetylation and Methylation
Modifications that typically occur on lysine residues, influencing gene expression and protein function.
Ubiquitination
The attachment of ubiquitin to lysine residues in proteins, tagging them for degradation.