OCR A 2.1.4 Acids
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
acids
proton donors
bases
proton acceptors
acid + water reaction

H3O+ name
hydronium ion
bases + water reaction
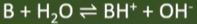
alkali
-soluble base (in water)
-produce OH- ions when added to water
weak acid
-carboxylic acid (CH3COOH)
-backward reaction favoured
-less H+ produced
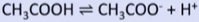
strong acid
-HCl, H2SO4, HNO3
-forward reaction favoured
-more H+ ions produced
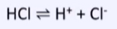
strong base
-NaOH, KOH
-forward reaction favoured
-more OH- produced
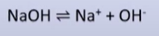
weak base
-NH3
-backward reaction favoured
-less OH- ions produced

acid and base reaction
-metal from base (NH4+ ion from ammonia) react w/ non metal from acid
-to make salt (pH neutral)
-H+ ions produced by acid react w/ OH- ion produced by base
-to make water
making standard solutions
-weigh out amount of solid precisely using balance and weighing boat
-transfer solid from weighing boat to beaker, wash solid left into beaker using deionised water
-dissolve solid fully w/ water; stir to ensure solid dissolved fully
-transfer sol to volumetric flask, using funnel to prevent spillage, rinse beaker & glass rod into flask to make sure most of sol is transferred
-use more of water to fill to graduation line using pipette
-invert flask few times w/ lid on to ensure thoroughly mixed

