WJEC AS Chemistry Unit 1.4 and 1.5 - Bonding and Solid Structures
1/43
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
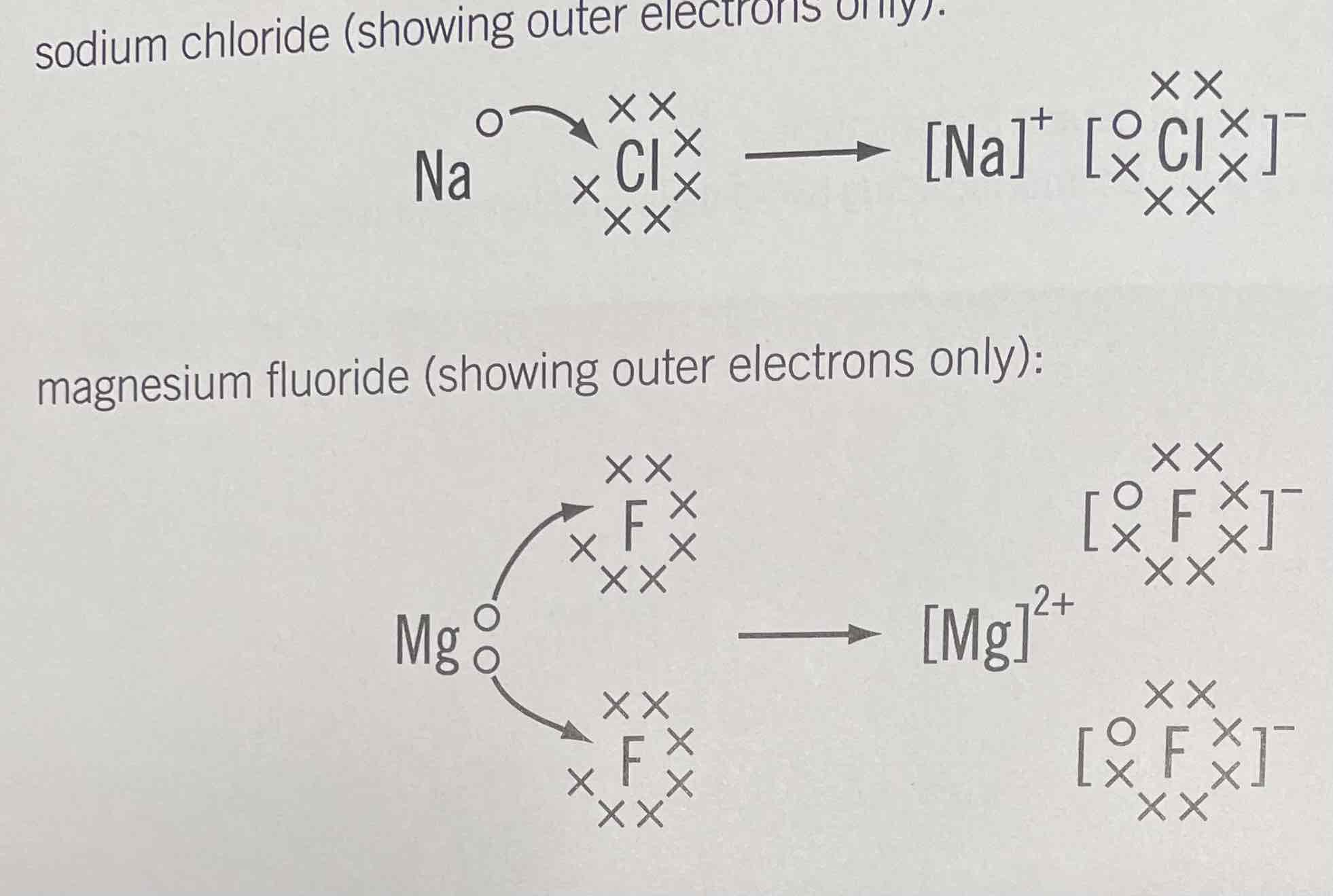
Ionic bond
Electrostatic attraction between oppositely charged ions
Metal + non-metal
Predominantly groups 1 and 2 with 6 and 7
Process of ionic bonding
metal atom loses electrons and becomes a cation (+ve)
Non-metal atom gains electrons and becomes an anion (-ve)
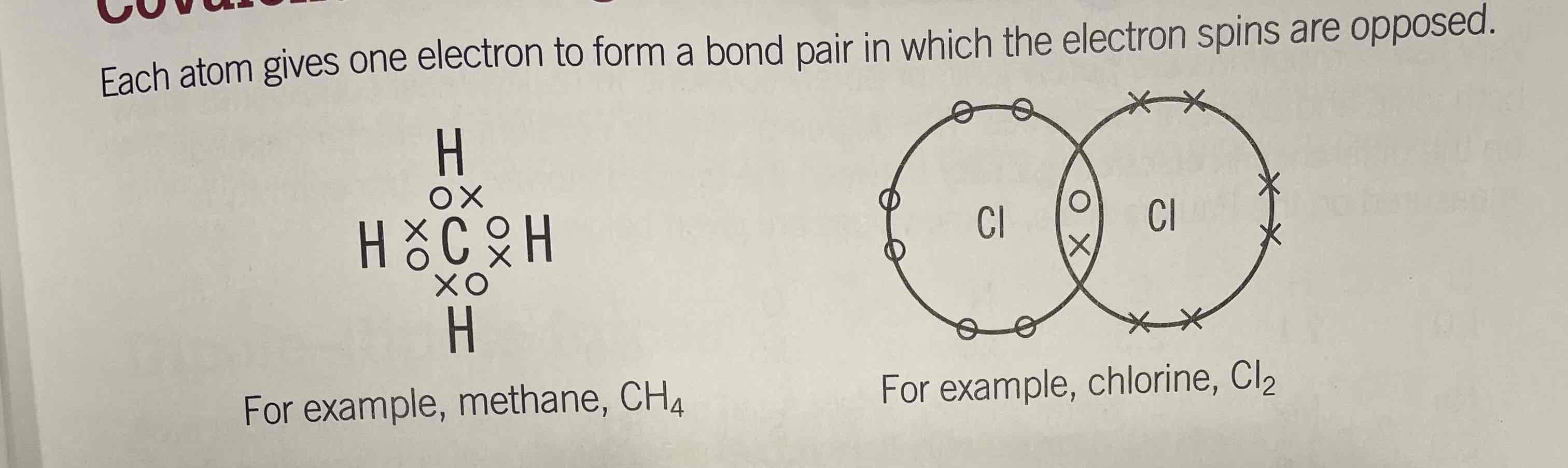
Covalent bond
pair of electrons shared between two atoms, with each atom giving one electron, forming a bond pair in which the electron ships are opposed
Non-metal + Non-metal
Includes diatomic molecules; HNFOICB
Forces of attraction and repulsion in covalent molecules
the electrons in the pair between the atoms repel one another but this is overcome but their attractions to both nuclei
If atoms get too close together the nuclei and inner electrons will repel those of the other atom, so the bond has a certain length
The electron spins must be opposite for the bonds to form
Forces of attraction and repulsion in ionic bonding
Cations and anions are arranged so that each cation is surrounded by several anions + vv to maximise attraction and minimise repulsion
Repulsions from inner electrons and nuclei prevent the ions from getting too close together
Coordinate bonding
Both electrons are contributed by the same atom
Intermediate character of ionic and covalent bonds
the degree of ionic and covalent character and properties depends on the difference in electronegativity between the bonded atoms
Electronegativity
The ability to attract electrons in a covalent bond
Higher electronegativity = better the element can attract bonding electrons
No units as relative value
N, O and F are the 3 most e.n. elements
F = most as high charge and small atomic radius
Depends on;
nuclear charge (e.n. is higher as proton number increases) therefore increases across a period
atomic radius (smaller = higher e.n.) a.r. decreases slightly across a period and increases down a group
group 8/0 not considered as does not participate in covalent bonding
Polar bonds
Polar = one end of bond with a slightly positive charge, other end with a slightly negative charge
bonding electrons are pulled towards more electronegative atom
Partial charged written above atom with symbol delta
Equal electronegativity = electrons equally shared = non-polar bond
Difference in e.n. < 0.4 = non-polar covalent bonds
Difference 0.4-1.9 = polar covalent bonds
Difference 2.0 or more = ionic
Intermolecular bonding
Weak bonding holding the molecules together
Governs the physical properties of the substance
Three types
Much weaker than covalent and ionic bonds
Intermolecular bonding
strong bonding between atoms in the molecules
Governs its chemistry
Dipole-dipole forces
Polar molecules have dipoles; one end slightly positive charge, one end slightly negative charge due to difference in e.n.s between the atoms in the molecule
There will be an attraction between them if they arrange them selves to that the negative region of one molecule is close to the positive region of another molecule
Forms a permanent dipole-dipole force
No dipole-induced dipole forces
A molecule with no dipole (symmetrical distribution of electron cloud)
The + end of the molecule can pull the electron cloud of a neighbouring molecules towards it, giving the left side of that molecule a - charge
Induced a temporary dipole in the neighbouring molecule
The two dipoles are attracted to each other
Temporary dipole-induced dipole interactions
A temporary dipole induces an induced dipole in a neighbouring molecule
van der Waals forces
Weakest form of intermolecular forces
Includes dipole-dipole and temporary dipole-temporary dipole forces
Induced dipole-Induced dipole and the effect on physical properties
Strength of forces increases with increasing number of electrons int he molecules
The more electrons in a molecule, greater the fluctuation in the electron cloud around the nuclei + the larger the temp + induced dipoles created
—> stronger forces between the molecule
Shown in the boiling temperatures of the noble gases or the halogens
Hydrogen bonding
Strongest intermolecular forces
Occur between molecules containing hydrogen atoms bonded to small, very electronegative elements which have lone pairs - Fluorine, Oxygen or Nitrogen
F, O + N = highly electronegative elements
The + charge in the bonded H atom is spread over a small volume + so has a high charge density
Highly Polarising + H atom then attracts a lone pairs of e-s from a small highly en atom in another molecule
Effect of hydrogen bonding on physical properties
Solubility;
the most significant IMFs between water molecules = H bonds
Covalent compounds that can replace these bonds by forming new H bonds with water will dissolve
Boiling Temperatures;
H bonds = strongest IMFs
Molecules that form H bonds have a higher boiling temperatures than molecules of a similar size that cannot H bond
VSEPR principle
The shape of a molecule or ion is governed by the number of pairs of electrons in the outer (valence) shell of the central atom
The electron pairs arrange themselves around the central atom as far apart as possible from each other to minimise repulsion between them
lp:lp repulsion > lp:bp repulsion > bp:bp repulsion
Use of the VSEPR Principle in predicting the shapes of simple molecules and ions

Geometry of molecules with two bond pairs around the central atom
The bps of e-s arrange themselves at 180 degrees to each other
Geometry; linear
Geometry of molecules with 3 bond pairs around the central atom
The bps = 120 degrees to each other
Trigonal planar
Geometry of molecules with 4 bond pairs around the central atom
109.5 degrees
Tetrahedral
Geometry of molecules with 5 bond pairs around the central atom
Three of the atoms are in a plane at 120 degrees to each other
The other two atoms are at 90 degrees to this plane
Trigonal bipyramidal
Geometry of molecules with 6 bond pairs around the central atom
90 degrees
Octahedral
Geometry of molecules with 3 bond pairs and 1 lone pair
The strong repulsion between an lp and a bp forces the bps together, slightly reducing the bonding angel between them to 107 degrees
Trigonal pyramidal
Geometry of molecules with 2 bond pairs and 2 lone pairs
lp:lp repulsion forced the bps closer together and reduce angel between them to 104.5 degrees
V-shaped
Simple structures
weak intermolecular forces
strong intramolecular forces
Examples; water, carbon dioxide, hydrogen gas
Gases or volatile (low bp) liquids
Covalent in groups 1, 2, 3, 5, 6, 7 (not 0 (full os) or 4 (giant covalent))
Giant structures
strong forces of attraction
Solids at room temperature
Ionic compounds adopt a giant structure
Group 4 covalent —> giant covalent (e.g. diamond and graphite)
Metallic —> giant structure
Ionic solids
Giant lattices of positive and negative ions
Structure of the crystal depends on the relative number of ions + their sizes
Made of the same base unit repeated
Ions are arranged so the electrostatic forces of attraction between the oppositely charged ions is greater than the electrostatic repulsion between ions with the same charge
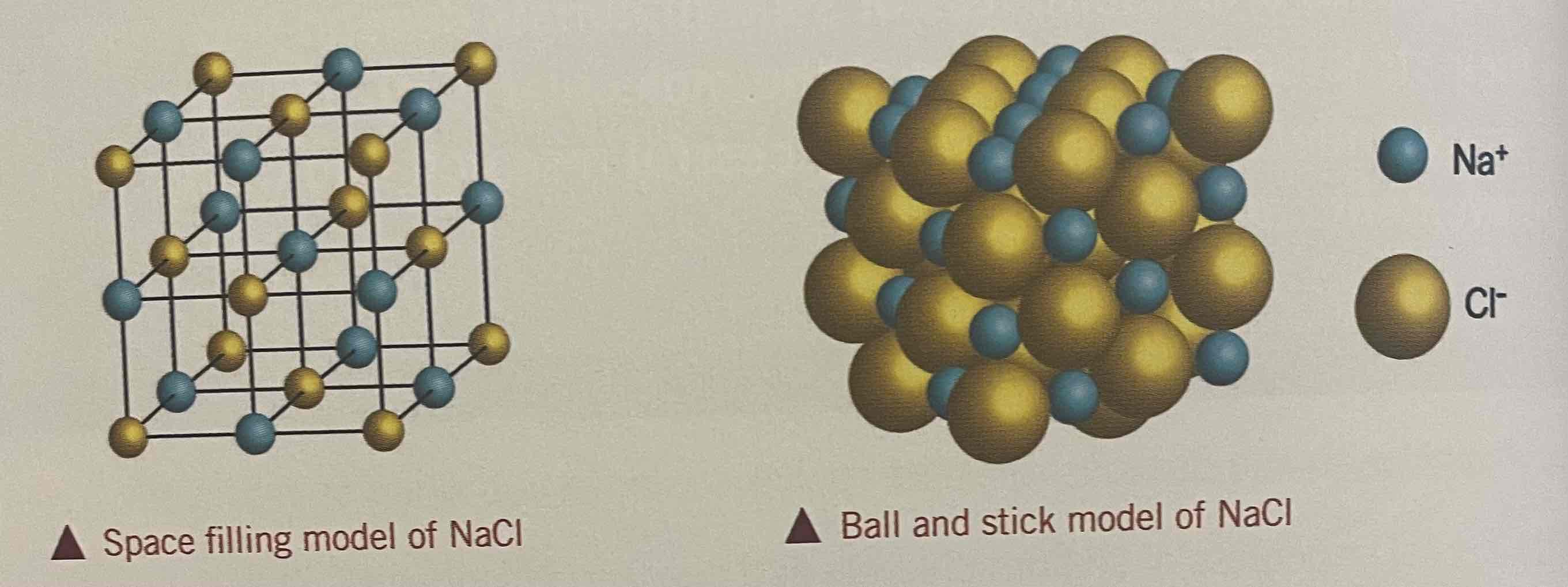
Sodium chloride
Consists of sodium ions and chloride ions
Each Na+ ion is surrounded by 6 Cl- ions + vv
Coordinate number of each ion = 6
Face centred cubic structure
Caesium chloride
Caesium ion is larger than the Sodium ion, therefore more Cl- ions can fit around it
Each Cs+ ion is surrounded by 8 Cl- ions + vv
Coordination number of each ion = 8
Adopts a body centred cubic lattice structure
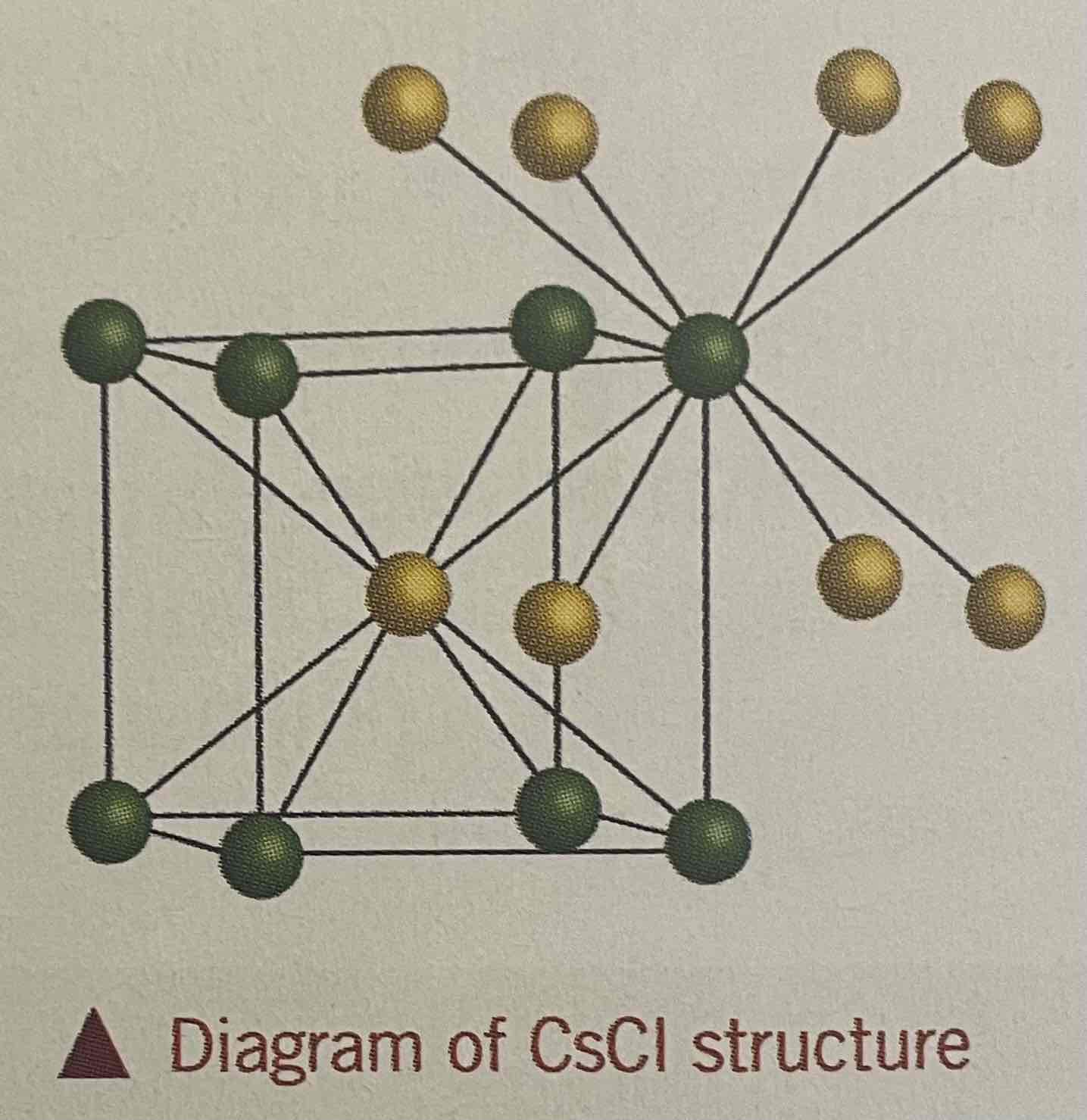
Reason for the difference in structure between sodium chloride and caesium chloride
Different cation size
rCs+ > rNa+ and both have a 1+ charge
Charge in Na+ is more concentrated due to smaller size
Electrostatic attraction is stronger in NaCl
Caesium is smaller than Sodium
Physical properties of ionic compounds
Relatively high melting points (higher than simple covalent, lower than giant covalent);
giant lattices are held together by strong esa between opp charged ions. Takes a large amount of energy to overcome these forces of attraction
Non-Conductors/poor electrical conductivity;
conduction requires moving negatively charged particles
In the solid state, ions are in fixed positions by the strong ionic bonds
Electrolytes;
When molten/dissolved, ions are free to move and will move conduct electricity
Most are soluble in water;
Water molecules = polar
Oxygen ends attracted to negative ions
Hydrogen ends attracted to negative ions
Like dissolved like; a charged solute dissolves in a charged solvent
Diamond
Giant tetrahedral structure
Each C atom is covalently bonded to 4 others
Bonding forces are uniform throughout the structure
Physical Properties of Diamond
Very high melting temperature; energy need to break strong c.b.s = very high
Extremely hard; strength of the c.b.s + the geometrical rigidity of the structure
Insoluble in water; no ions to attract the polar water molecules
Poor conductor of electricity; no delocalised electrons or ions present
Graphite
Hexagonal layer structure
Each layer; C is joined to 3 others by strong cobalt. Bonds
4th electron from each Carbon atom = delocalised within the layer
Hexagonal layers held together by weak induced dipole-induced dipole forces
Physical properties of Graphite
Very high melting temperature; strong c.b.s in the hexagonal layers
Soft + slippery; weak forced between layers easily broken, layers can slide over each other
Insoluble in water; no ions to attract the polar water molecules
Good conductors of electricity; delocalised electrons are free to move along the layer so electricity can flow. Delocalised electrons not free to move one layer to the next so it can only conduct parallel to its layers
Low density; relatively large amount of space between layers so length of c.b.s is much shorter than the length of the vdW forced between the layers
Physical properties of giant molecular substances
Very high melting temperature due to strong covalent bonds
Insoluble in water as there are no ions (sometimes no delocalised electrons) to attract the polar water molecules
Iodine
Solid; molecules held in a lattice by weak intermolecular forces
Atoms covalently bonded in pairs to form diatomic iodine molecules
Held together by weak can der Waals forces
Ice
Giant tetrahedral structure containing stronger intermolecular hydrogen bonds
Molecule of water arranged in rings of six
Water molecules = further apart than they are in the liquid state. The structure —> large areas of open space inside the rings + therefore ice = less dense than liquid water
Metallic bonding
Electrostatic attraction between delocalised electrons and the nucleus of the cations
Metals = lattice
Physical properties of metals
High melting temperatures; larger energy needed to overcome stronger forces of attraction between the nuclei of the metal cations and the delocalised electrons. Melting affected by number of dl e-s per cation + the size of the cation
Hard; metallic bond is very strong
Insoluble in water; no ions to attract the polar water molecules
Good conductors of electricity in both solid and molten state; delocalised electrons can carry electricity
Good thermal conductors; delocalised electrons can pass KE to each other
Malleable + ductile; when a force is applied to a metal the layers of cations can slide over each other
Physical properties of simple molecular substances
Low melting and boiling temperatures; intermolecular forces are weak and don’t need much energy to break
Soft; weak IMFs between the molecules are easily broken
Normally insoluble in water; no ions to attract the polar water molecules. Compounds that can form H bonds with water will be soluble
Poor conductors of electricity; do not contain delocalised electrons or ions