Required practical 8- Dehydrogenase activity in chloroplasts
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
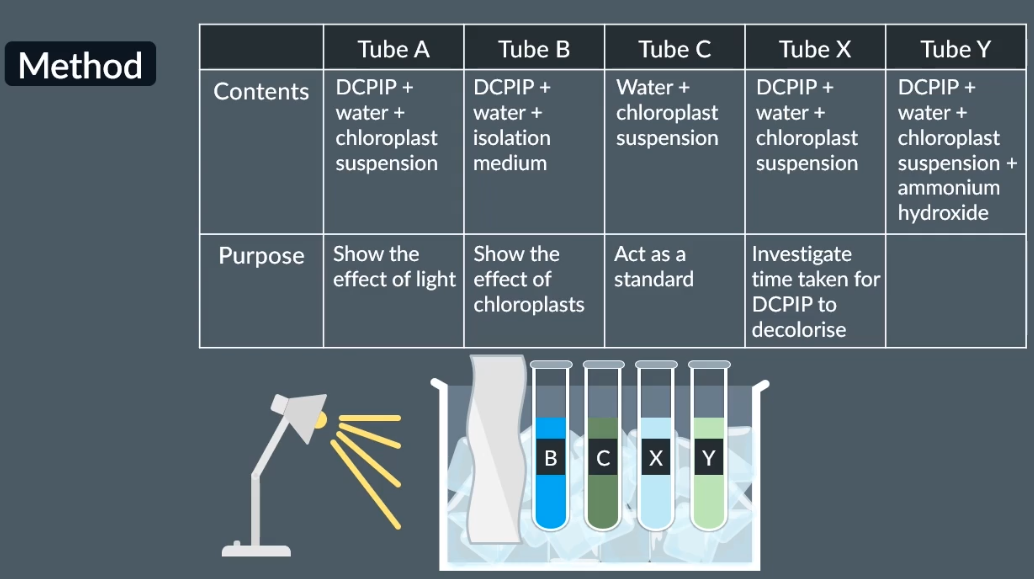
Outline the method to prepare chloroplast suspension:
Place a small beaker in a larger beaker half filled with ice.
In another beaker add some iosolation medium and crushed spinach leaves
Then get a funnel add three layers of muslin and some drops of iololation medium and pour into the small beaker placed inside the ice beaker
Outline the method for testing the effect of dehydrogenase activity in chloroplasts:
For tube y the purpose is to investigate the effect of ammonium hydroxide
So, without ammonium hydroxide it takes shorter time to decolorise
With ammonium hydroxide it takes longer time to decolarise, reducing the rate of dehydrogenase enzyme
When removing the foil for tube A, which appears a lighter shade, shows that both light and chloroplasts are required for this reaction
The idea behind the practical
Dehydrogenase enzyme catalysts the reaction NADP + H → NADPH during the light dependent reaction
So to find the effect of ammonium hydroxide on the time taken for DCPIP to decolorise (from blue to colourless)
When it becomes colourless the DCPIP is reduced
So, DCPIP accepts the electrons instead of (NADP)
So reduces the rate of dehydrogenase enzyme
Practical implications:
When blending the spinach leaf, so not put the midrib or the stalk into the blender, as they are tough to blend, and they contain little amount of chloroplasts
Muslin is used as filter paper, as it removes the large debris, but allows organelles such as chloroplast through
Isolation medium: contains same water potential, buffer, and cold
So, less osmotic activity, no denaturing of enzymes and minimal enzyme activity
End point is subjective, leading to inaccurate results being recorded, to overcome this make sure to use a colorimeter