GABA & Glutamate
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
65 Terms
What makes GABA distribution different to most other NTs
GABA is widely and uniformly distributed throughout the brain
This is in contrast to most other neurotransmitters which have a
localised, discrete distribution (e.g. acetylcholine, noradrenaline, dopamine and serotonin)
In GABA transmission, what is taken into the neuron, how?
Glutamate via carrier mediated transport
How does Glutamate become GABA
Glutamate —(glutamic acid decarboxylase)-→ GABA
GABA is actively packaged into vesicles by an specific transporter & is released via what mechanism
classical Ca2+ -mediated exocytosis
Termination of GABA is via what
uptake by a GABA transporter
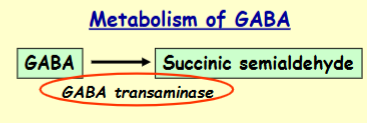
Degradation of GABA is via what
Degradation is via GABA transaminase
GABA exerts its effects via how many subtypes of receptor?
Name them
2 main subtypes
GABAA and GABAB receptors

GABAA receptors are permeable to what
Cl-
Name the 5 binding sites on GABAA receptors & when their ligand binds do they enhance/inhibit
GABA binding site - agonists/antagonists can bind here
Benzodiazepine binding site - enhance the actions of GABA
Barbiturate binding site - enhance the actions of GABA
Neurosteroid binding site - enhance the actions of GABA
Picrotoxin binding site - blocks the Cl- channel

True/False GABA transmission is always inhibitory
True - GABA is the main inhibitory transmitter in the brain

GABA is required for what functions
General CNS depression (not the mood disorder)/inhibition
Regulates/modulates the activity of other neurotransmitter systems
What makes Glutamate distribution different to most NTs
Glutamate is widely and uniformly distributed throughout the brain
This is in contrast to most other neurotransmitters which have a localised, discrete distribution (e.g. acetylcholine, noradrenaline, dopamine and serotonin)
In Glutamate transmission, what is taken into the neuron, how?
Glutamine is taken into the neuron via carrier mediated transport
What converts glutamine → Glutamate
Glutamine —(Glutaminase)→ Glutamate
Glutamate is actively packaged into vesicles by an specific transporter & is released via what mechanism
Release is via classical Ca2+ - mediated exocytosis
How is glutamate terminated & degraded
Termination is via uptake by a glutamate transporter
Degradation is via glutamine synthase
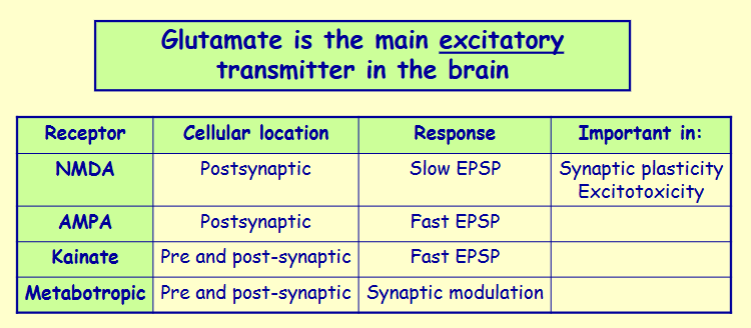
Glutamate exerts its effects via 4 main subtypes of receptor - name them
NMDA, AMPA, kainate and metabotropic receptors
Important
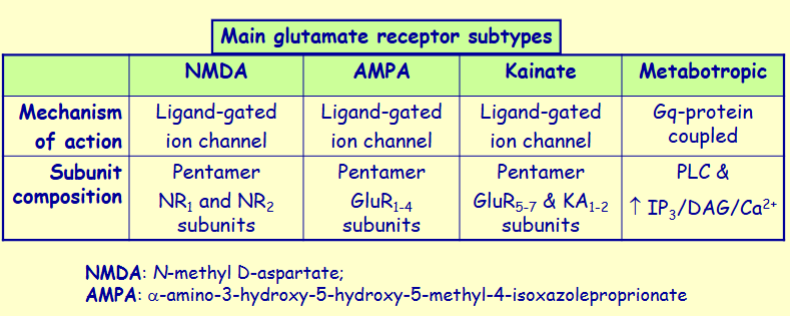
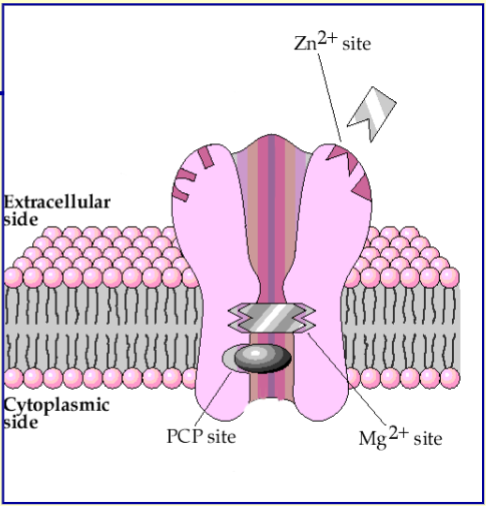
What is unique about the ions that NMDA receptor is permeable to
permeable to Na+, Ca2+ and K+
Ca2+ permeability here is important

Name the 3 inhibitory sites on NMDA receptors
Mg2+ site - channel is normally blocked by Mg2+ when the cell is normally polarised but is overcome when the cell is depolarised
Zn2+ site - binding of Zn2+ inhibits receptor opening
Channel blocking drug site - certain drugs (e.g. PCP selectively block the channel)
Is Glutamate excitatory/inhibitory
Glutamate is the main excitatory transmitter in the brain
EPSP: Excitatory post-synaptic potential
Glutamate is required for what functions
General CNS excitation/activation
Regulates/modulates the activity of other neurotransmitter systems
In what 2 treatments is the glutamate receptor used
Head injury and stroke - A major drug target is the development of glutamatergic antagonists to reduce excitotoxic brain damage following head injury and stroke
Epilepsy - Some anti-epileptic drugs work by antagonizing glutamate receptors, specifically the AMPA subtype (e.g. perampanel)
Epilepsy is a ______ disorder characterised by what
neurological disorder characterised by seizures
What causes seizures
episodic high-frequency discharge of a group of neurons in the brain
Where in the brain do seizures happen
Can happen in any region - usually starts locally, but then spreads to other areas of the brain
The symptoms depend on the region of the brain affected
Prevalence of epilepsy (%)
0.5-1% are affected
In most cases of epilepsy , there is no recognisable cause, but 2 possible causes could be
After brain damage (trauma, infection, tumours)
In certain inherited neurological disorders
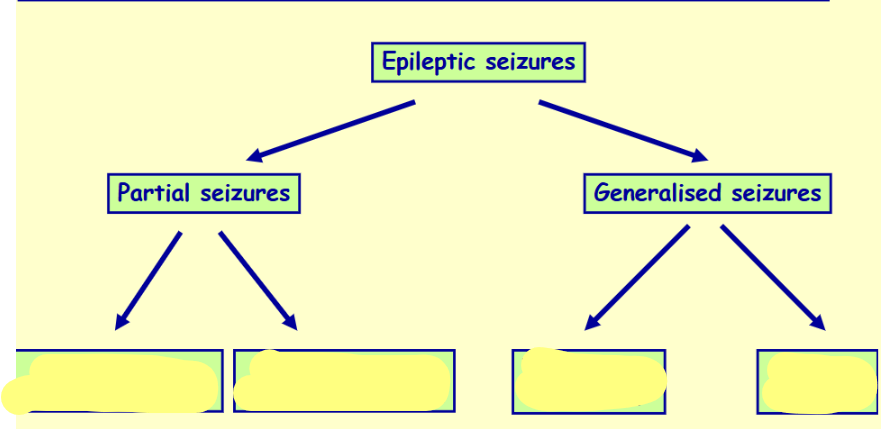
Name & differentiate these 4 types of epilepsy

What happens in the tonic phase of tonic-clonic seizures
An initial strong contraction of the whole musculature
Rigid extensor spasm
Respiration may stop
Defecation, micturition and salivation may occur
What happens in the clonic phase of tonic-clonic seizures
A series of violent synchronous jerks
How long does the clonic phase last
2-4 minutes
After what phase does the patient regain consciousness after tonic-clonic seizures & in what state are they
Patient recovers consciousness feeling ill and confused after the clonic phase
What happens in absent seizures
The patient abruptly stops whatever he or she was doing and stares vacantly for a few seconds
The patient is unaware of his or her surroundings
In what state do people recover from absent seizures
They recover abruptly with little after-effects
What age group is most affected by absent seizures
Children
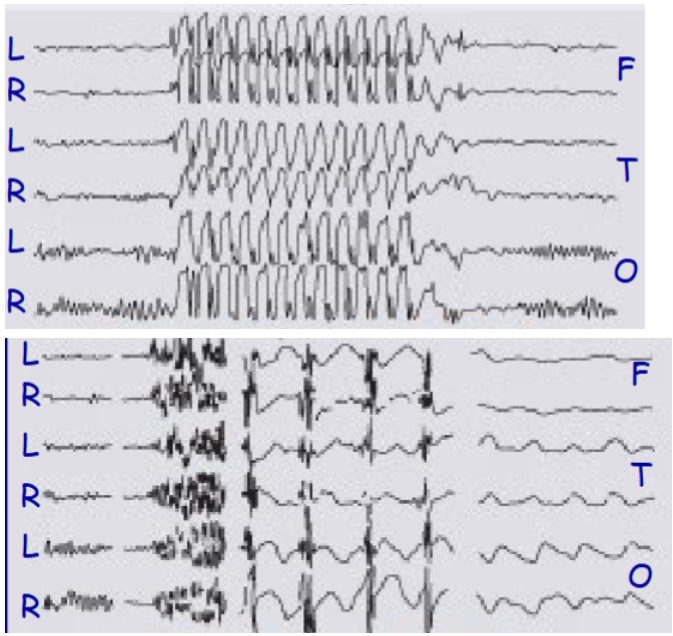
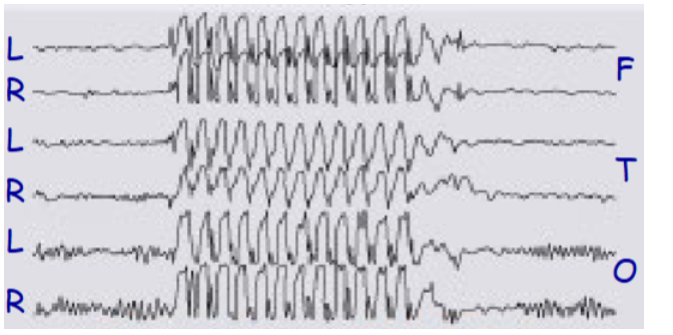
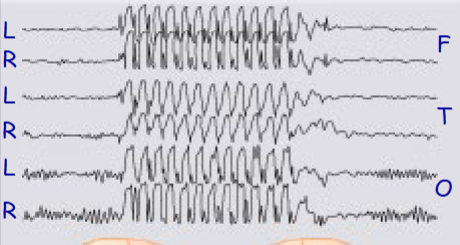
which one of these represents an EEG of a tonic-clonic seizure or an absent seizure
Top = absent
Bottom = tonc-clonic

Describe the tonic-clonic seizure EEG
1 – normal discharge, 2 – tonic phase, 3 – clonic phase, 4 – post-seizure coma
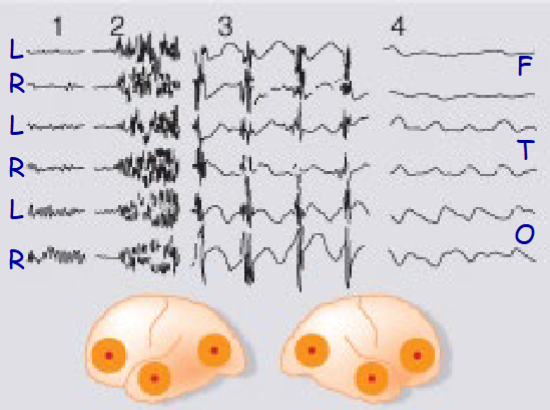
Describe the EEG pattern of absence seizures
EEG pattern reflects neural oscillations between thalamus and cortex and is due to T type calcium channels
Describe the neurochemical basis underlying seizures (glutamate, GABA, electrical properties)
- Enhanced excitatory amino acid (glutamate) transmission
- Reduced inhibitory amino acid (GABA) transmission
- Abnormal electrical properties of the affected cells
Risk associated with repeated epileptic seizures
Repeated epileptic discharge can cause neuronal death through excitotoxic mechanisms
Name a form of epilepsy that is associated with neurodegeneration
Lennox-Gastaut syndrome is a particularly severe form of epilepsy that affects children. It is associated with progressive mental retardation which probably occurs as a result of neurodegeneration.
Antiepileptic/Anti-convulsant drugs are fully effective in treating seizures in what % of patients
50-80%
Name the 4 main long-established anti-epileptic drugs
Phenytoin
Carbamazepine
Valproate
Ethosuximide
(others include Barbituates (e.g. phenbarbital) & Benzodiazepines (e.g. diazepam, clonazepam, lorazepam))
By what 4 mechanisms do anti-epileptic drugs control abnormal discharge
- Enhancement of GABA action
- Inhibition of voltage-gated sodium channel function
- Inhibition of voltage-gated calcium channel function
- Antagonism of glutamate receptors
Aim of anti-epileptic treatment
Prevent abnormal discharge whilst leaving normal discharge intact
Some anti-epileptic drugs work by enhancing GABAergic transmission. Give 3 mechanisms by which this would work & name a drug for each mechanism
- Positive allosteric modulation of the GABAA receptor (e.g. barbituates and benzodiazepines)
- Inhibition of GABA transaminase (e.g. vigabatrin)
- Inhibition of GABA uptake (e.g. tiagabine)
How do Benzodiazepines enhance GABAergic transmission
Benzodiazepines bind to the GABAA receptor at a different site to GABA and increase the affinity of GABA for the receptor
Mechanism of action of phenytoin, carbamazepine, valproate & lamotrigine
Inhibiting voltage-dependent sodium channel function thereby reducing neuronal membrane excitability → prevents propagation of action potentials
Phenytoin, carbamazepine, valproate & lamotrigine blocking action shows the phenomenon of use dependence - explain
They preferentially block the excitation of neurons that are firing repetitively)
They preferentially bind to the inactivated state of the Na + channel
Valproate can work by inhibiting voltage-dependent sodium channel function or ….
inhibiting T-type voltage-gated calcium channel function that underpins absence seizures
What other anti-epileptic drug (other than valproate) works by inhibiting T-type voltage-gated calcium channel function that underpins absence seizures
Ethosuximide
How do gabapentin and pregabalin work as anti-epileptic drugs
They work by binding to a subunit of P/Q-type voltage-gated calcium channels thereby preventing it from trafficking to the membrane. This reduces calcium dependent exocytosis of synaptic vesicles.
How does perampanel work as an anti-epileptic drug
By antagonizing glutamate receptors, specifically the AMPA subtype
5 elements of normal fear response to threatening stimuli
- Defensive behaviours
- Autonomic reflexes
- Arousal & alertness
- Corticosteroid secretion
- Negative emotions
Name & explain 6 clinically recognised anxiety disorders
Generalised anxiety disorder (ongoing state of anxiety with no clear reason)
Social anxiety disorder (fear of being/interacting with other people)
Panic disorder (attacks of overwhelming fear in association with marked somatic symptoms – sweating, tachycardia, chest pains, trembling, choking etc.)
Obsessive compulsive disorder (compulsive ritualistic behaviour driven by irrational anxiety)
Phobias (strong irrational fears of specific things or situations)
Post-traumatic stress disorder (anxiety triggered by insistent recall of past stressful experiences)
Main type of anxiolytic drugs
Benzodiazepines (e.g. diazepam (Valium®); alprazolam (Xanax®)
Anxiolytic drugs can also be drugs used to treat other things give 5 examples
1) Some drugs used for depression (e.g. SSRIs such as fluoxetine (Prozac®))
2) 5-HT1A receptor agonists (e.g. buspirone)
3) Beta-adrenoceptor antagonists (i.e. beta-blockers such as propranolol)
4) Some drugs used for epilepsy (e.g. gabapentin, pregabalin etc.)
5) Some drugs used for schizophrenia (e.g. olanzapine, risperidone etc.)
Name the 4 types of benzodiazepines & give 2 examples of each
Ultrashort duration
- *Midazolam
- *Zolpidem (Ambien®) (not strictly a benzo, but similar MoA)
Those 2 are mainly used as hypnotics - sleeping pills)
Short duration
- Lorazepam
- Temazepam
Medium duration
- Alprazolam
- Nitrazepam
Long duration
- Diazepam (Valium®)
- Chlordiazepoxide
Name 5 pharmacological effects of benzodiazepines
Reduction of anxiety and aggression - useful for acute anxiety states, behavioural emergencies, certain medical, surgical and dental procedures
Sedation and induction of sleep - useful for transient/acute causes of sleep disturbance such as jet lag, emotional upse
Reduction of muscle tone and coordination
Anticonvulsant effects - useful for epilepsy including life-threatening status epilepticus
Anterograde amnesia - prevent formation of memories of events experienced while under their influence. (Flunitrazepam (Rohypnol®); the “date-rape” drug)
True/False Benzodiazepines can’t be taken for a long time
True - only recommended for short durations as tolerance/dependence can occur, as well as rebound insomnia