Topic 4: Halogens
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
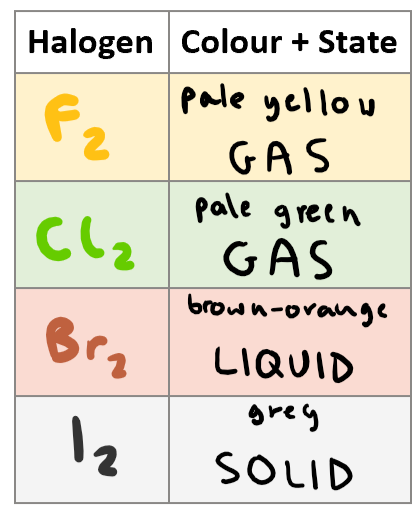
From fluorine to iodine, mention the state and the colour.
What is the trend for boiling point down the halogen group? Explain this

What is the trend for electronegativity and reactivity down the halogen group? Explain this

What is the trend for oxidising agent power down the halogen group? Explain this

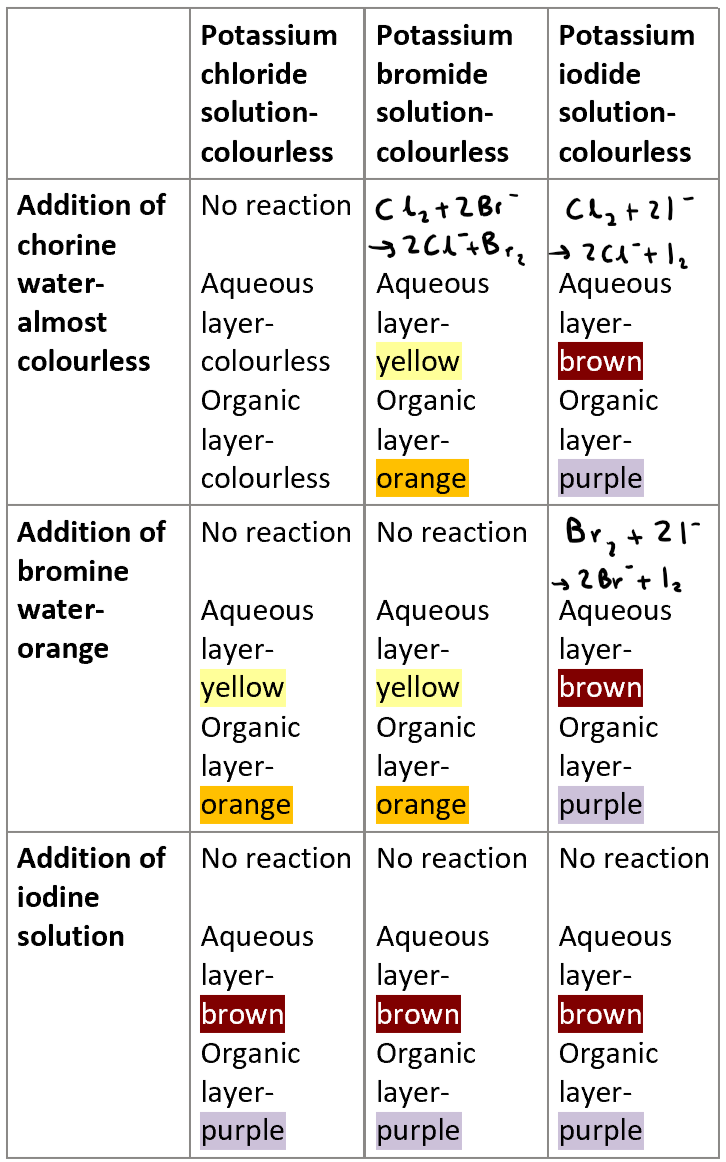
When you react a potassium halide with any halogen, what are observations you’ll have?
Aqueous layer and organic layer
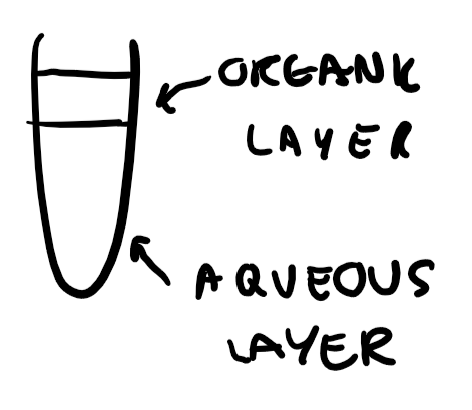
What is the colour of any potassium halide solution?
Aqueous layer- colourless
Organic layer- colourless
Reaction between KBr and Cl2? Also mention the colour of aqueous layer and organic layer
2KBr + Cl2 → 2KCl + Br2

Reaction between KI and Cl2? Also mention the colour of aqueous layer and organic layer
2KI + Cl2 → 2KCl + I2

Reaction between KI and Br2? Also mention the colour of aqueous layer and organic layer
2KI + Br2 → 2KBr + I2

What is the general trend when reacting a potassium halide with a halogen?
A halogen will displace a halide from solution is the halide is lower in the periodic table
What are the 3 disproportionation reactions halogens have?
1) Halogen + cold alkali
2) Halogen + hot alkali
3) Chlorine + water
What is the reaction of a halogen with a cold alkali (NaOH for an example)? Give the general and ionic equation + include oxidation numbers
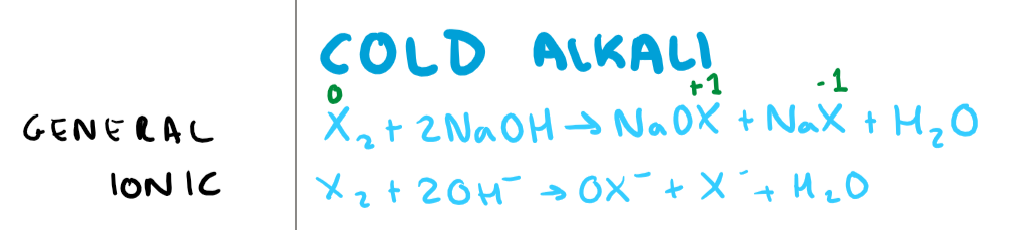
What is the reaction of a halogen with a hot alkali (NaOH for an example)? Give the general and ionic equation + include oxidation numbers
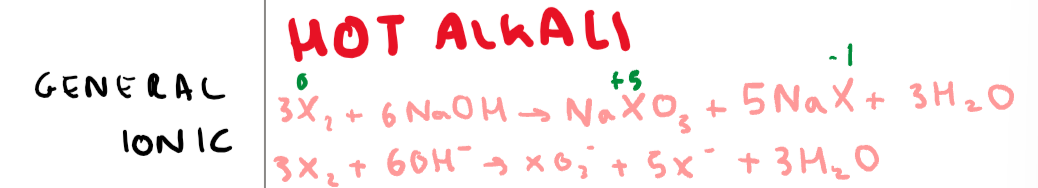
How do you make bleach?
Use cold sodium hydroxide, also NaClO (bleach)
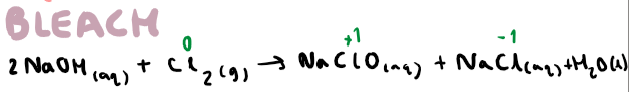
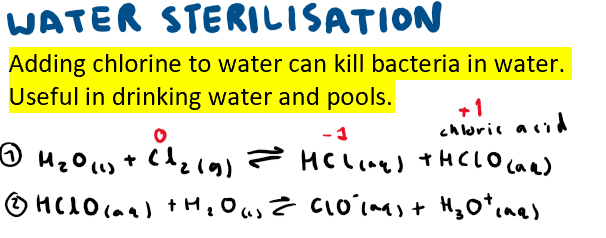
Show the equations for the sterilisation of water + include oxidation numbers
What halide is the best to use to reduce sulfuric acid?
Iodide ions (NaI or KI)
Write the equation(s) for potassium chloride and sulfuric acid + observations (include oxidation numbers)
White misty fumes from (HCl)
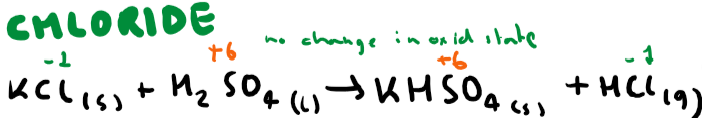
Write the equation(s) for potassium bromide and sulfuric acid + observations (include oxidation numbers)
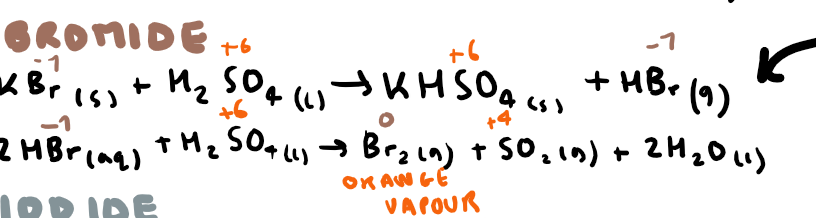
Write the 3 equations for potassium iodide and sulfuric acid + observations (include oxidation numbers)
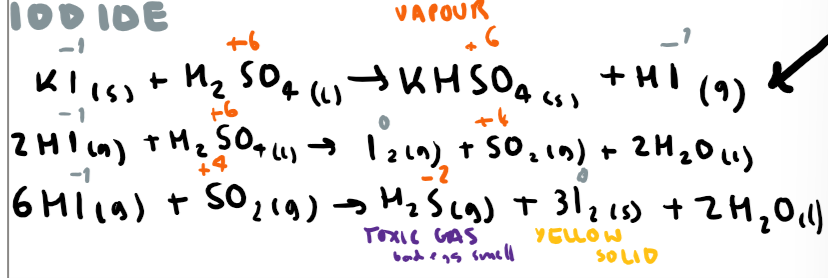
Write the 4 equations for potassium iodide and sulfuric acid + observations (include oxidation numbers)

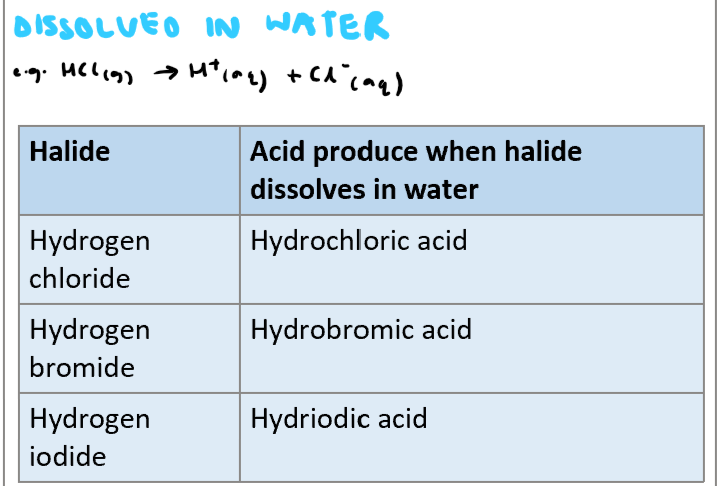
Write the general equation for dissolving hydrogen halides in water and also list the acids produced when HBr, HCl and HI are dissolved in water.
Write the equation for hydrogen halide reaction with moisture in the air and note any observations

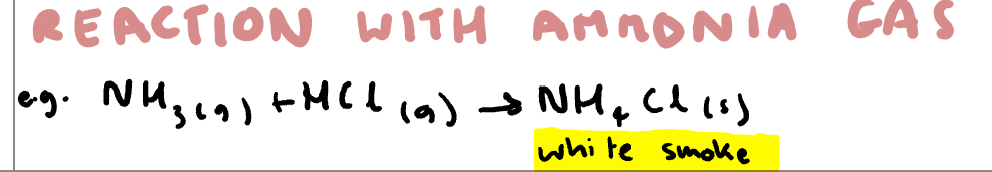
Write the equation for hydrogen halide reaction with ammonia gas and note any observations