Optical Isomerism
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
"How do you draw a molecule in 3D?"
Predict the shape of the molecule:
Identify the central atom and count the number of bonding electron pairs around it.
For example, if there are 4 bonding pairs of electrons, the shape will likely be tetrahedral.
predict the bond angles (109.5).
Draw the molecule in 3D
Use dashed lines to represent bonds going away from you (into the page).
Use wedge lines to represent bonds coming toward you (out of the page).
Use straight lines to represent bonds in the plane of the page.
"What does it mean when a carbon atom is described as asymmetric?"
When a carbon atom is bonded to 4 different groups then we say it is asymmetric
"When is a carbon atom considered symmetric?"
If the carbon is bonded to fewer than 4 groups or if 2 of the groups are the same then we say it is symmetric
‘Asymmetric’ carbon atoms
We can take an asymmetric carbon atom alongside its 3D model and show that it is impossible to draw a line of symmetry - in the diagram above there are 2 different molecules on either side of the molecule
"How can we visualize symmetry in a molecule with a carbon bonded to 2 same groups or an atom bonded to 3 or fewer groups?"
we can take a carbon which is bonded to 2 groups which are the same or an atom that is bonded to 3 or less groups, reveal their 3D models and show that we can draw a line of symmetry
"What makes a molecule chiral, and what do we call a carbon atom in a chiral molecule?"
If a molecule contains an asymmetric carbon atom then we sya that the molecule is a chiral molecule. As a result, instead of calling them asymmetric carbon atoms, we can also call them chiral carbons
more complex chiral molecules
A chiral molecule can contain any number of chiral centres (atom which has 4 different bonds). But a chiral molecule must contain at least one chiral centre
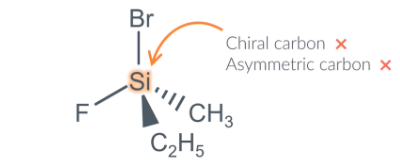
what is this molecule
This molecule is also a chiral molecule because it contains a chiral centre BUT the chiral centre is not a carbon atom so we cant say it contains a chiral carbon or asymmetric carbon
When drawing the mirror image of a molecule in 3D:
If your given a 2D molecule remember to first draw it in 3D
draw a mirror plane.
draw the chiral centre.
draw on the atom closest to the mirror plane.
repeat step for the 3 remaining atoms.
check all bonds and atoms for accuracy.
drawing mirror images of molecules
The letter C is closet to the dotted line so we make sure to draw that in our mirror image which explains why the COOH looks strange
What is the enantiomer of this molecule?
We say that a chiral molecule and its mirror image are enantiomers. Enantiomers are optical isomers of each other (because they have the same bonds and structural formulas but different arrangement of atoms in space)
The formal definition of enantiomers is
To draw the enantiomer of any chiral molecule:
draw the displayed formula for the molecule.
identify the chiral centre.
draw a 3d Depiction of the molecule.
draw the mirror image of the chiral molecule
Ch3
H
Ch2CH3
fill in the rest
"Why aren’t optical isomers considered a form of structural isomerism?"
Optical isomers aren’t a form of structural isomerism because optical isomers have the same structural formula
"How are E/Z isomers similar to optical isomers?"
EZ isomers are similar to optical isomers since both relate to molecules with the same structural formula but with a different spatial arrangement of atoms.
So, they are both types of stereoisomerism
"How are E/Z isomers different from optical isomers?"
EZ isomers are different to optical isomers since Optical isomers contain chiral centres, whereas EZ isomers have none.EZ isomers are superimposable, whereas optical isomers are not.
Isomers Tree Diagram
From front-on, the movement of a wave is shown as a straight line
How does a side on light wave look
At chemistry a level we assume a light wave from side on look like this
How does a front on light wave look
A light wave look like this from front on
unpolarised light vs plane polarised light
"What is plane-polarized light?"
If light waves are arranged at the same angle, we say that they’re plane polarised light. Plane polarised light is the term that will be used in the exams
"What is unpolarized light?"
If light waves aren’t arranged at the same angle , we say that they’re unpolarised light
"How can we get plane-polarized light from unpolarized light?"
We can get plane polarising light by shining unpolarized light through a polarising filter
"How do enantiomers interact with plane-polarized light?"
Enantiomers can interact with light and so we say that they’re optically active. One type of enantiomer will rotate plane polarised light clockwise. The other will rotate plane polarised light anticlockwise.
Which of these show light that has been shone through an enantiomer
Solutions on non chiral molecules don’t rotate light. The highlighted green shows rotated light which means it was shown through an enantiomer
Which of these mixtures is a 50:50 split of enantiomers
A 50:50 mixture of enantiomers does not rotate plane polarised light (so they are optically inactive)
What happens when a racemic mixture is formed instead of a single enantiomer, and how does it interact with light?
In a lab, it is challenging to produce a solution with only one enantiomer. Instead, we often create a racemic mixture containing both enantiomers of a chiral molecule. These mixtures interact with light differently than solutions with a single enantiomer
For example E1 rotates light clockwise. If we compare it to mixture of E1+E2 the light will move slightly anticlockwise and if the 2nd mixture contains higher proportion of the E2 then it will move further anticlockwise
What is a racemic mixture, and how does it affect plane-polarized light?
A 50:50 mixture of enantiomers is known as a racemate or a racemic mixture.
These mixtures don’t rotate plane polarised light so therefore we say that they’re optically inactive .