AQA GCSE C1 Atomic Structure
1/57
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
What is the definition of an element?
An element is a substance made up of only one type of atom.
What is a group on the periodic table?
A group is a column on the periodic table.
What is a period on the periodic table?
A period is a row on the periodic table.
What is a compound?
A compound is different types of atoms bonded together.
What does a chemical bond do in a compound?
A chemical bond holds atoms tightly together in compounds.
What is the difference between a molecule and a compound?
A molecule is two or more atoms joined together chemically. A compound is two or more different elements joined together chemically.
A molecule is not a compound. A compound is a molecule.
What is a reactant?
Reactant - the substances you start with in a reaction.
What is a product?
Product - the new substances made in a reaction.
What is a balanced equation?
Balanced Equation - same number of each type of atom on both sides of the equation.
State the law of conservation of mass.
Atoms cannot be created or destroyed in a chemical reaction.
Total mass of products formed in a reaction is equal to the total mass of the reactants.
What are the state symbols?
The State Symbols:
Solids - (s)
Liquids - (l)
Gases - (g)
Aqueous Solutions, substances dissolved in water - (aq)
What is a mixture?
A mixture is made up of two or more substances that are not chemically combined together.
What are the techniques available to separate mixtures?
The techniques available to separate mixtures:
Filtration
Crystallization
Distillation
Chromatography
What is filtration?
Filtration - separates insoluble substances in a solvent from the soluble substances in the solvent.
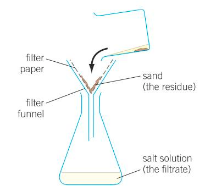
Describe crystallisation/evaporation following filtration.
Heat the solution in an evaporating dish on a water bath.
Stop heating at the point of crystallization (when small crystals appear)
Leave the rest of the water to evaporate at room temp - a crystallization dish can be used (flat bottom) to give a large surface area for evaporation.
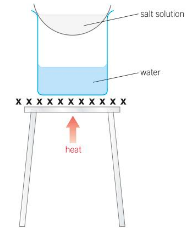
Why use a water bath during evaporation?
It is gentler to evaporate solution when using a water bath.
What are the differences between compounds and mixtures?
What is Distillation?
Distillation is the act of separating a soluble solid from a solvent and collecting the solvent rather than allowing it to evaporate.
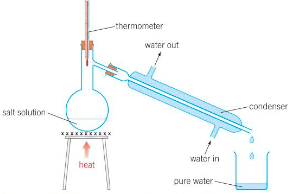
Describe Distillation.
Distillation:
A solution is heated and boiled to evaporate the solvent.
The vapour given off enters a condenser (an outer glass tube with water flowing through it), cooling the inner glass tube and condensing the vapour.
The hot vapour is cooled and collected after condensing, the newly condensed substance being collected in a receiving vessel. Any dissolved solids remaining in the heated flask.
What is Fractional Distillation?
Fractional Distillation is the act of separating mixtures of miscible liquids, such as ethanol and water (dissolve in each other, mixing completely).
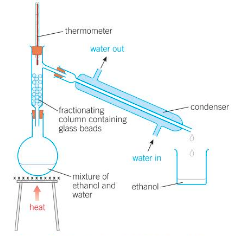
Describe fractional distillation.
Fractional Distillation:
Uses fractionating column - a tall glass column with glass beads in it.
The temp is highest at the bottom of the column, getting lower as you go up.
The substance with the highest boiling point will condense quicker going up the column, and will drip back down to the flask beneath.
The lower boiling point liquid will keep going up the column and do regular distillation.
What is Paper Chromatography?
Paper Chromatography is used to separate (and identify) substances.
Paper Chromatography works because some compounds in a mixture will dissolve better than others in the solvent chosen.
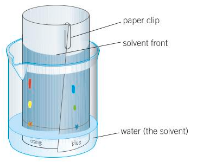
Describe Paper Chromatography.
Paper Chromatography:
A capillary tube is used to tab a spot on a pencil line near the bottom of the sheet of absorbent chromatography paper (which is placed standing in a solvent at the bottom of a beaker or tank).
The solvent soaks up the paper, running through the spot of mixture.
The spot of mixture will travel up the paper as it soaks - how far it travels up the paper is dependent on the solubility of the mixture (the more soluble the substance, the further up the paper it’d go.
Who were the first to have ideas about particles and atoms?
The ancient Greeks were the first to have ideas about particles and atoms.
Who suggested that substances were made up of atoms that were like tiny, hard spheres? When was this? What else did he suggest?
John Dalton suggested that substances were made up of atoms that were like tiny, hard spheres in the 1800s.
He also suggested that each chemical element had its own atoms that differed from others in their mass. He believed these atoms could not be divided or split.
Who discovered the electron? When was this?
At the end of the 1800s, J.J. Thomson discovered the electron.
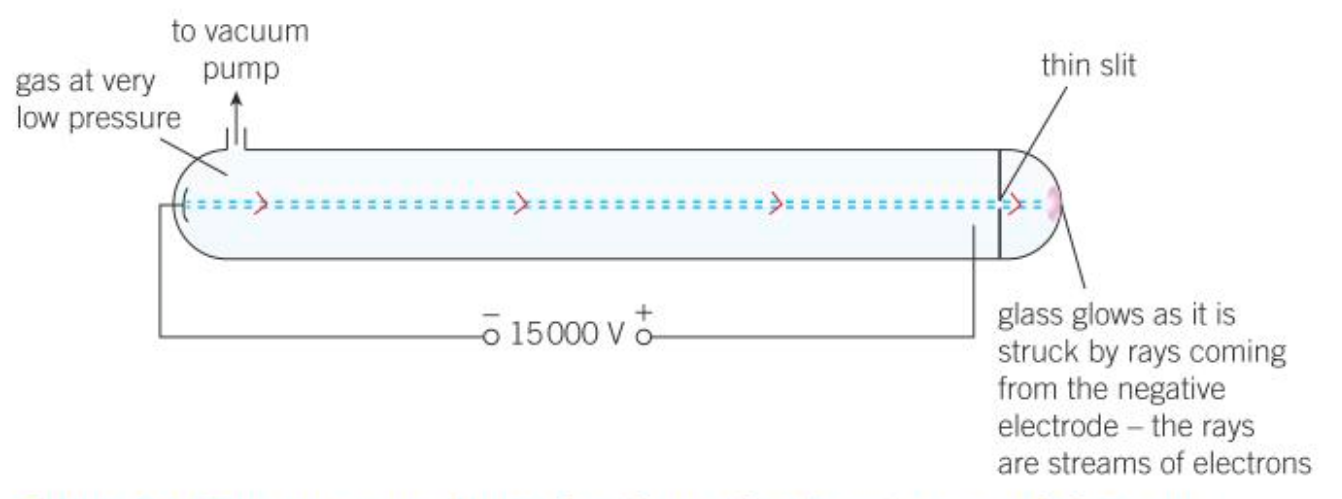
How was the electron discovered?
J.J. Thomson discovered the electron when experimenting by applying high voltages to gases at low pressure.
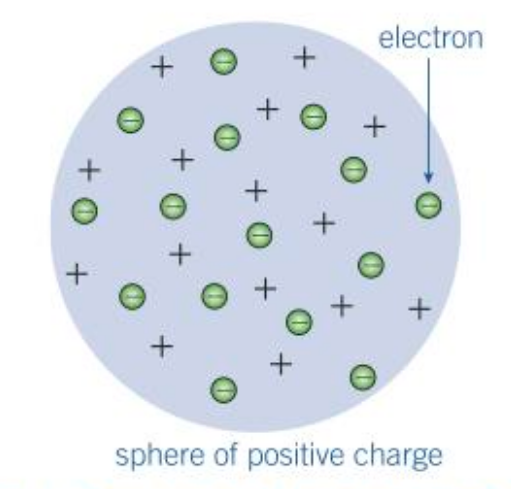
Who came up with the plum pudding model and why?
J.J. Thomson said that the tiny negatively charged electrons must be embedded in a cloud of positive charge. He knew that the atoms themselves carry no overall charge, so any charges in an atom must balance out. He imagined the electrons as the bits of plum in a plum pudding.
Who disproved the plum-pudding model and how?
Geiger and Marsden were firing dense, positively charged radioactive alpha particles at a very thin piece of gold foil. They expected the particles to pass straight through the gold atoms as the gold atoms were supposedly a diffuse cloud of positive charge. However, some of the atoms were repelled back.
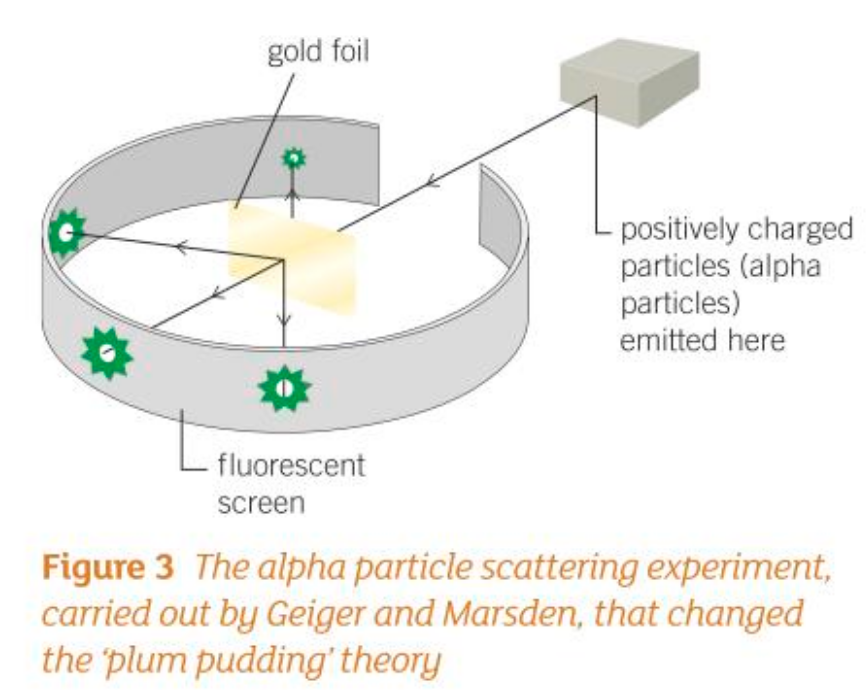
Who suggested that Thomson’s atomic model was not possible based off of Geiger and Marsden’s experiment?
Rutherford suggested that Thomson’s atomic model was not possible. He said that the positive charge must be concentrated at a tiny spot in the centre of the atom. Otherwise, the large, positive particles fired at the foil could never be repelled back towards their source.
It was proposed that the electrons must be orbiting around this nucleus which contains very dense positively charged protons.
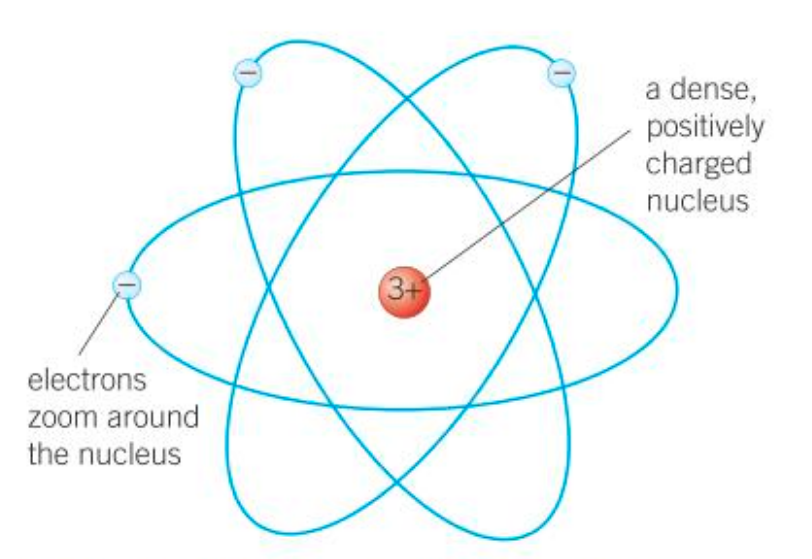
Who suggested that the electrons must be orbiting the nucleus at set distances, in certain fixed energy levels (shells)?
Niels Bohr noticed that the light given out when atoms were heated only had specific amounts of energy. He suggested that the electrons must be orbiting the nucleus at set distances, in certain fixed energy levels (or shells).
The energy must be given out when excited electrons fall from a high to a low energy level.
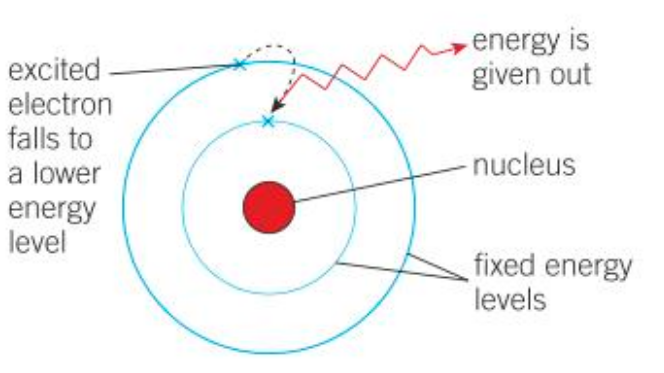
Who discovered the neutron? When?
In 1932, James Chadwick did an experiment that could only be explained by the existence of neutrons.
What is the relative charge and relative mass of each type of sub-atomic particle?
What is the atomic number of an element?
The atomic number of an element is the number of protons in the atom of an element.
What is the mass number of an element?
The mass number of an element is the number of protons plus neutrons in the nucleus of an atom of an element.
What is the current periodic table arranged in?
The modern periodic table is arranged in order of their atomic number.
What is an ion?
An ion is a charged atom.
What type of ion would you have if you lost an electron? What if you gained an electron?
If an atom lost an electron, the atom would become a positive ion.
If an atom gained an electron, the atom would become a negative ion.
Example:
O (oxygen) loses an electron, it becomes O(+)
O (oxygen) gains 2 electrons, it becomes O(2-)
How would you know the mass number and atomic number of an element?
What is an isotope?
Isotopes are atoms of the same element with different numbers of neutrons.
What property do isotopes sometimes have?
Isotopes are unstable and therefore sometimes radioactive.
What is different between different isotopes of an element?
Samples of different isotopes of an element have different physical properties.
Example:
Different density
What is the same between different isotopes of an element?
Different isotopes of an element always have the same chemical properties, because their reactions depend on their electronic structures.
Which shell has the lowest energy level? Which shell do electrons occupy?
The lowest energy level is shown by the shell that is nearest to the nucleus.
Electrons in an atom occupy the lowest available energy level.
How many electrons can each shell hold?
The first shell can hold up to 2 electrons.
The second energy level/shell can hold up to eight electrons.
The third shell can hold up to eight electrons.
What does the chemical properties of an element depend on?
The chemical properties of an element depend on how many electrons it has in its highest energy level (outermost shell).
Therefore, elements in the same group will react in a similar way as they all have the same amount of electrons in their outermost shell.
what are the basic physical properties of metals?
strong but malleable
good conductors (of both heat and electricity)
high boiling and melting points
Transition Metals - properties
typical metal properties
can have more than one ion (eg copper can be Cu+ and Cu+2)
often form coloured compounds
often make good catalysts
Group 1 - properties
known as alkali metals
soft and low density
very reactive
Group 1 - trends
As you go down:
increasing reactivity - outer electron more easily lost
lower melting and boiling points
Group 1 - reaction with water
produce a metal hydroxide and hydrogen gas
reactions are violent, potassium releases enough energy to combust
Group 1 - reaction with chlorine
form white metal chloride salts
violent reactions
Group 1 - reactions with oxygen
produces a metal oxide
Group 7 - properties
known as halogens
diatomic
very reactive
low melting/boiling points
Group 7 - trends
As you go down:
less reactive
higher melting/boiling points
Displacement Reactions
more reactive halogens will displace less reactive halogens in compounds
eg. chlorine can displace bromine in an aqueous solution of potassium bromide
Group 0 - properties
known as noble gases
inert, colourless gases
Group 0 - trends
As you go down:
boiling points increase as there are more electrons meaning greater intermolecular forces to be overcome