NSAIDs
1/76
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
77 Terms
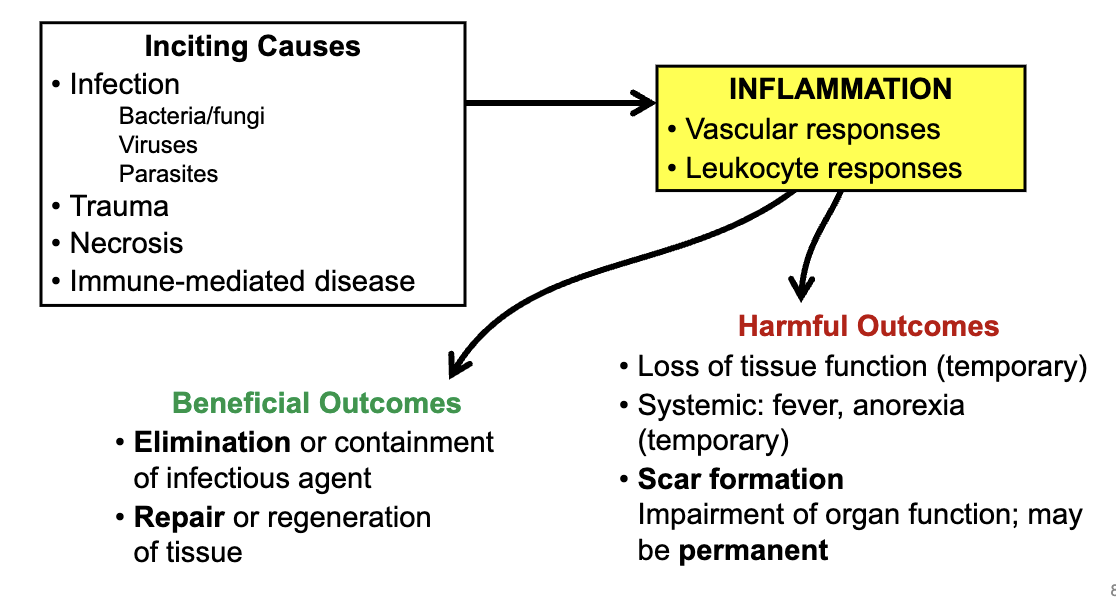
what is inflammation
the active response of tissues to injury that can be either protective & beneficial or exaggerated & harmful
local response at the site of injury '
complex response
inflammation is a complex response which involves
immune response
coagulation cascade
regeneration & repair processes
function of inflammation
to protect the body following injury
removal of injurious stimuli (bacteria, chemical irritants, etc)
removal of necrotic cells
containment of damage (e.g. abcessation)
stimulation of repair & regeneration
what are the 4 major changes that occur in inflammation
1) blood vessels dilate (warmth & redness)
2) blood vessels become leaky (fluid & proteins enter tissue → edema)
3) WBCs enter inflamed tissue
4) Nociceptors become sensitized (pain)
cardinal signs of inflammation
heat
redness
swelling
pain
loss of function
upon tissue injury/infection, leukocytes rapidly produce ___________ that effect changes on blood vessels and tissues
inflammatory mediators
eicosanoids are (mostly) pro-inflammatory mediators:
prostaglandins
thromboxane
prostacylin
leukotrienes
what do the most effective anti-inflammatory drugs inhibit
many or all of the pro-inflammatory mediators
synthesis or inflammatory mediators
some are produced in advance for rapid release at time of insult or injury
e.g. histamine exists pre-formed inside cells
how are eicosanoids synthesized
at the site of tissue injury in response to the injury to trigger the inflammatory response and recruit immune cells
redundancy?
several mediators will trigger the same inflammatory process, so inhibitors of one class of mediator may lessen, but not abolish, inflammation
in many cases, ___________ will alleviate the inflammation
eliminating the insult
in some cases, an exaggerated inflammatory reaction to a mild or harmless stimulus does more harm than good
e.g. allergies, autoimmune reactions, etc
chronic inflammation stimulates
fibrosis (scarring)
depending on the site, may impair
vision, motility, oxygenation, or cause seizures, arrhythmias, intestinal strictures, etc
anti-inflammatory therapy may be necessary if
stimulus cannot be identified or eliminated
inflammation summary
non-pharmacological options to reduce inflammation
ice & heat
elevate
lifestyle habits
pharmacological options to reduce inflammation
NSAIDs
glucocorticoids
aspirin MoA
inhibition of prostaglandin synthesis
what is the oldest NSAID
aspirin
what are NSAIDs
a family of chemically dissimilar drugs that produce three main benefits
three main benefits that NSAIDs produce
anti-inflammatory effects
antipyretic effects (fever)
analgesic effects
clinical uses of NSAIDs
for the relief of musculoskeletal & inflammatory pain, including post-operative pain
what are prostaglandins
PGs are eicosanoids that exhibit diverse roles in inflammation and cellular signalling
what does phospholipase A release upon stimulation
arachidonic acid from the plasma membrane
what enzymes then synthesize PGs and other eicosanoids from arachidonic acid
Cyclo-oxygenase (COX) enzymes
what two main COX enzymes exist in humans
COX-1 and COX-2
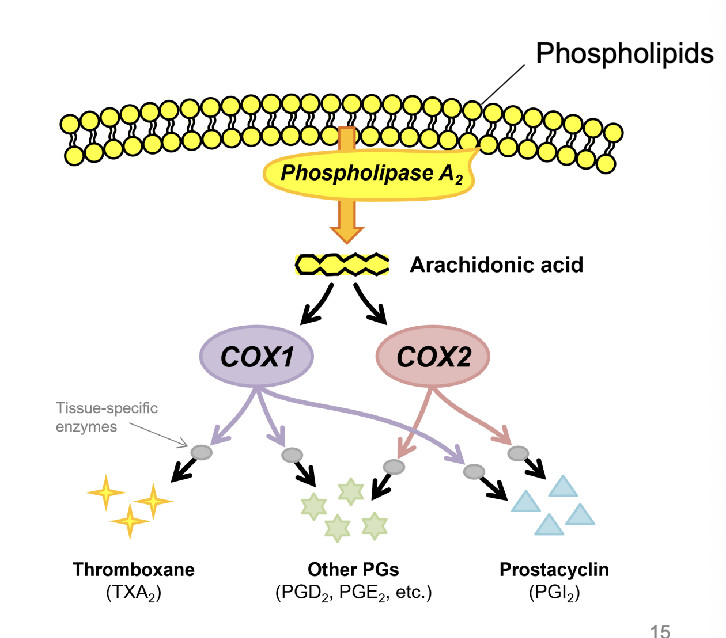
in the absence of inflammatory stimulus, COX-1
is a normal ‘housekeeping’ enzyme present at low levels in most tissues
in the absence of inflammatory stimulus, COX-2 is
normally present at much lower levels in most tissues, but is important for homeostasis in a few tissues (e.g. renal medulla, gastric mucosa)
thromboxane
synthesized by COX1 in platelets
promotion of platelet aggregation
other PGs (PGD2. PGE2)
maintenance of tissue blood flow
many other tissue-specific protective functions
prostacyclin (PGI2)
inhibition of platelet aggregation
vasodilation
other protective functions
PGE2 and PGI2 also involved in
gastric mucosa protection:
decrease acid secretion by gastric parietal cells, increase bicarb & mucus secretion, increase vasodilation
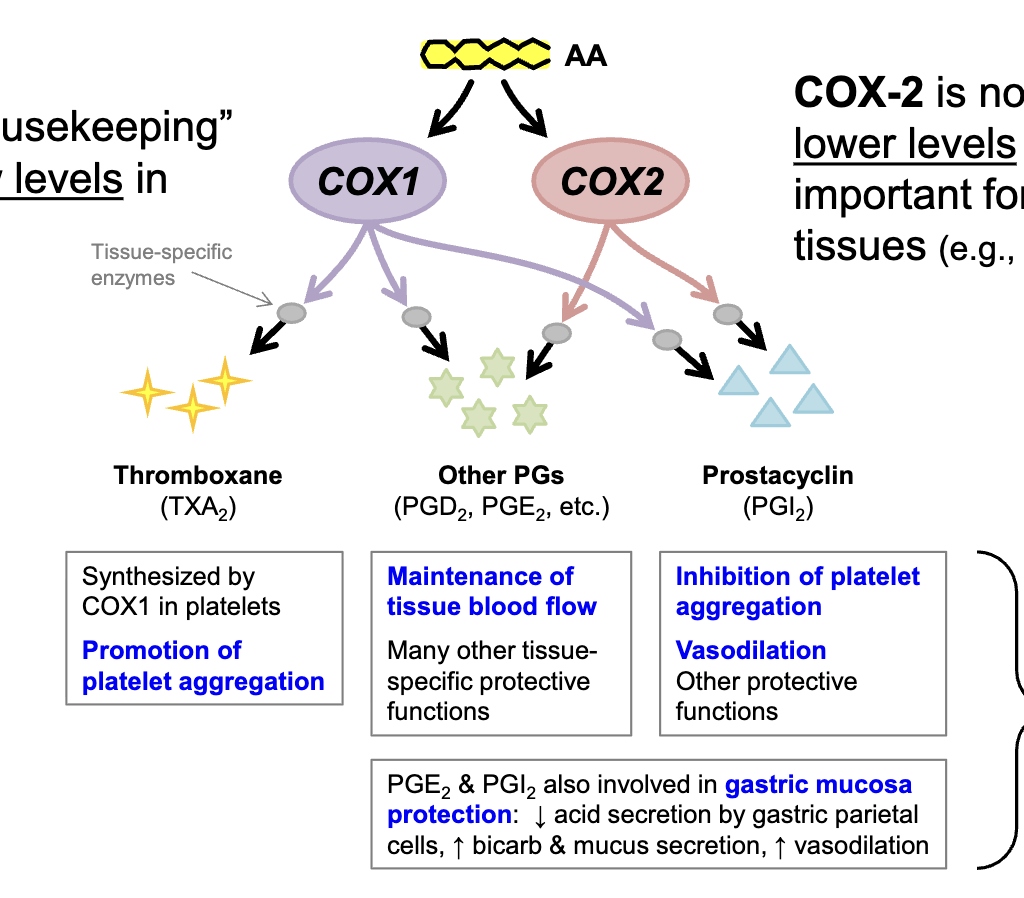
in the presence of inflammatory stimulus
COX-2 is upregulated in response to plasma membrane damage or inflammatory mediator release
COX-2 induction is a
local response that occurs at the site of cell damage or mediator release
excessive vasodilation occurs, promoting what
inflammation
redness
swelling
heat
pain
loss of function
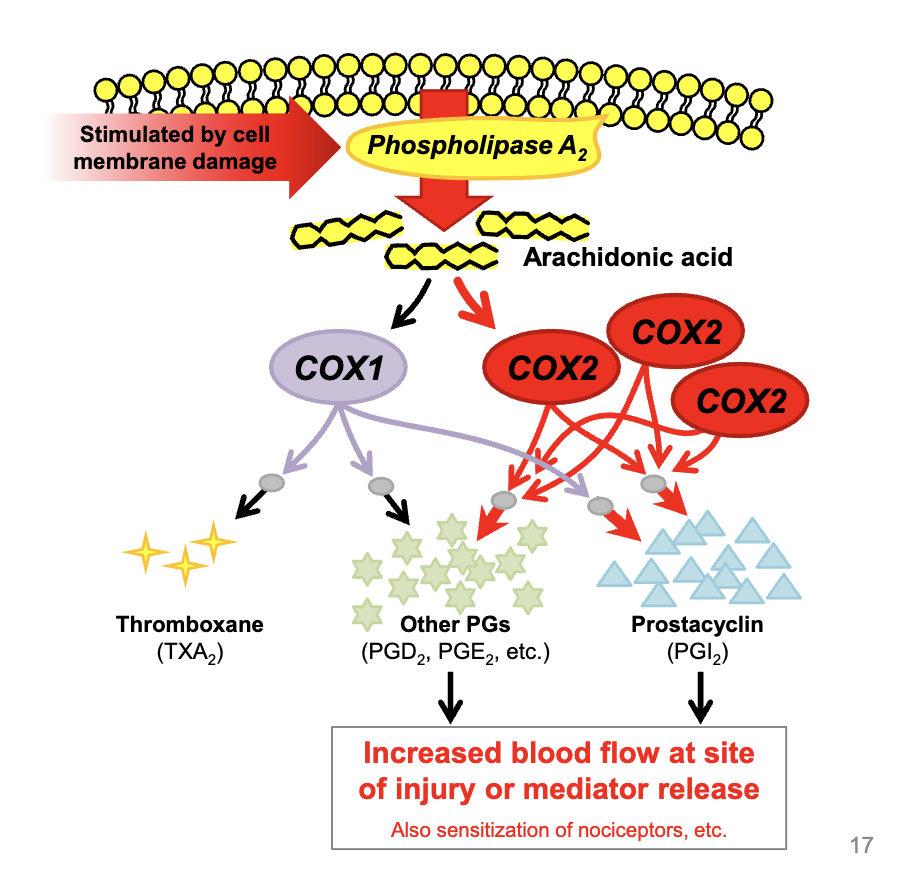
COX-1: constitutively expressed
always expressed, this enzyme consistently makes thromboxane and other prostaglandins required for normal maintenance, regardless or inflammation status
COX-2 inducibly expressed
this enzyme is only expressed at high levels to produce more prostaglandins and prostacyclin when inflammation is present
most NSAIDs inhibit
both COX1 & COX2
NSAIDs mechanism of action
NSAIDs inhibit cyclooxygenase enzymes
most NSAIDs inhibit both COX1 and COX2, what does this do
reduces synthesis of PGs, including those that promote vasodilation
reduces blood flow to site
reduces sensitization or nociceptors
alleviates inflammation
major benefit of NSAIDs is
a reduction in blood flow to the site of injury
adverse effects of NSAIDs
adverse effects in gastric mucosa
adverse effects in the kidney
adverse effects on platelets
adverse effects in gastric mucosa
the normal protective effects of PGs in the stomach are inhibited, resulting in
decreased blood flow, bicarb secretion, & mucus secretion
increased acid secretion
This can cause gastric bleeding with or without ulceration (the most common adverse effect associated with NSAIDs)
adverse effects in the kidney
COX enzymes produce PGs that maintain adequate blood flow to many tissues, including the renal medulla
excessive COX inhibition can lead to renal medullary hypoxia & papillary necorsis
in contrast to other tissues, in which COX-1 is the main homeostatic cyclooxygenase, COX-2 is particularly important in maintaining adequate blood flow in the renal medulla
adverse effects on platelets
only COX1 is present in platelets
NSAIDs inhibit the conversion of AA to thromboxane in platelets
result is a slightly increased general tendency to bleed
excessive doses of NSAIDs can cause more pronounced bleeding
(this effect can be used in our advanatage, e.g. low dose aspirin is used chronically in humans ro reduce myocardial infarction and stroke risk by inhibiting platelet aggregation)
mechanism of adverse effects in GI epitelium
excessive inhibition of PG synthesis in GI epithelium
decrease PGI2 → decrease blood flow → increase acid secretion, decrease bicarbonate secretion → gastric ulcers
decreased PGE2, decreased gastric mucus → gastric ulcers
mechanism of adverse effects in the kidney
excessive inhibition of PG synthesis in the kidney
decreased PGE2, decreased blood flow → hypoxia; renal papillary necrosis
mechanism of adverse effects in platelets
increased bleeding
decreased TXA2, decreased platelet aggregation → less clotting, increased bleeding
NSAIDs share similar
PK properties, adverse effects, and contraindications
PK properties
weak acids; dissolve best in stomach (~100% absorption)
primarily albumin bound/accumulate in cells at site of infection
extensive hepatic metabolism
efficient renal excretion
variable half-lifes (<6 hours: ibuprofen; >10 hours: naproxen)
adverse effects of NSAIDs
GI ulceration and stomach bleeding
inhibition of platelet aggregation → increased bleeding (most likely with aspirin)
inhibition of PG-mediated renal perfusion; renal papillary necrosis in dehydrated pts
inhibition of uterine motility
in humans, serious adverse events occur at rate of
~0.75 per million NSAID doses, and most are associated with overdosing
contraindications of NSAIDs
pts who are hypersensitive to ASA, salicylates, non-steroidal anti-inflammatory drugs (NSAIDs), analgesic, antipyretics
acute gastrointestinal ulcer
history of gastrointestinal ulcer
hemmorhagic diasthesis
active or severe hepatic failure, renal failure, or congestive heart failure
pts with a history of asthma induced by the administration of salicylates or substances with a similar action
combination with methotrexate at doses of 15mg/weel or more
late trimester of pregnancy
aspirin
acetylsalicylic acid
MoA of aspirin
Inhibits COX-1 and COX-2
is the inhibition of COX-1 and COX-2 reversible?
irreversible
(acetylates the COX 1 enzyme, destroying its activity)
are the effects long lasting
prolonged effects even at low doses
effects of aspirin
anti-inflammatory, antipyretic, analgesic
what is aspirin effective for
fever, musculoskeletal and cutaneous pain, but poor for visceral (internal organ) pain
what does aspirin do
inhibits COX-1 in platelets for their entire lifetime (10 days) → reduced platelet aggregation
Ibuprofen
derivative of propionic acid
what does ibuprofen do
inhibits both COX-1 and COX-2
is inhibition reversible
inhibition is reversible
effects of ibuprofen
anti-inflammatory, analgesic, antipyretic
when is advil recommended
arthritis, musculoskeletal pain, smooth muscle pain (maybe)
main adverse effect of ibuprofen
gastric ulceration but less intense than with aspirin → preferred for some chronic uses (arthritis)
celecoxib
almost 100% effective for COX-2
Celecoxib is approved for
osteoarthritis
does it provide analgesia
poor to negligible analgesia
does celecoxib effect platelets
no effect on platelets/bleeding because COX-2 not involved in thromboxane synthesis
is celecoxib likely to cause GI ulceration
far less likely to cause GI ulceration & bleeding than non-selective NSAIDs (if gastric lesions are not already present (COX2 products are involved in the healing of gastric ulcers)
main concerns with COX-2 inhibition
reduced blood flow to kidneys and intravascular blood clotting
increased risk of stroke & myocardial infarction
a concern with chronic use in patients with arthritis (osteoarthritis and rheumatoid arthritis), not with acute or intermittent use
how do Coxibs increase the risk of stroke and heart attack
regular non-selective NSAIDs inhibit the synthesis of all COX-1 and -2 products, both pro-and anti- clotting. Thromboxane is a potent pro-clotting molecule (promotes platelet aggregation), and its inhibition produces a greater effect than the inhibition of prostacyclin, so the net effect is slightly in favour of bleeding
Coxibs inhibit only COX-2, so less PGI2 is produced than usual, but thromboxane synthesis is not inhibited.
this tips the balance in favour of intravascular coagulation and therefore, stroke and myocardial infarction.
these effects are only observed after prolonged (>18 months) use

Acetaminophen
inhibits PG synthesis centrally (in CNS) → antipyretic, analgesic
little peripheral activity, so negligible anti-inflammatory effect & no effect on blood clotting
no longer considered an NSAID