A2 INORGANIC CHEMISTRY AND ANALYSIS
1/122
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
123 Terms
What is a transition element?
Definition: A transition element is a d-block element that forms one or more stable ions with an incomplete d subshell.
Where they are found: Between Groups 2 and 13 in the Periodic Table.
Exceptions:
Scandium (Sc) is not a transition element because Sc3+ has an empty 3d subshell.
Zinc (Zn) is not a transition element because Zn2+ has a completely filled 3d subshell.
How does the shape of a 3dxy orbital compare to the 3dz² orbital?
3dxy Orbital:
Four lobes positioned between the x and y axes.
Similar shape to the dxz and dyz orbitals, but oriented differently.
3dz² Orbital:
Dumbbell-shaped lobes along the z-axis.
A doughnut-like ring around the center.
What are the key properties of transition elements?
Variable oxidation states → Can form multiple stable ions.
Act as catalysts → Participate in redox reactions, stabilizing intermediates.
Form complex ions → Bond with ligands to create coordinate compounds.
Form coloured compounds → Due to d-electron splitting in ligand fields.
Why do transition metals exhibit variable oxidation states?
Reason: Incomplete d subshells allow electrons to be lost from both 3d and 4s orbitals.
Example:
Iron (Fe): Forms Fe2+ and Fe3+.
Manganese (Mn): Forms Mn2+, Mn4+, Mn6+, and Mn7+.
Why are transition metal compounds coloured?
Splitting of d-Orbitals
In free transition metal ions, the five d-orbitals have the same energy.
When ligands surround the metal ion in a complex, these orbitals experience an electric field and split into two energy levels:
Higher energy set (eg orbitals)
Lower energy set (t₂g orbitals)
This splitting depends on:
Type of metal ion
Oxidation state
Type of ligand
Geometry of the complex (e.g., octahedral, tetrahedral)
Absorption of Light & Electron Excitation
An electron in a lower-energy d-orbital can absorb visible light and move to a higher-energy d-orbital.
The wavelength absorbed corresponds to a specific colour of light.
The remaining wavelengths are transmitted or reflected, making the compound appear coloured.
Complementary Colours
The colour observed is the complementary colour of the light absorbed.
Factors Affecting Colour
Oxidation State of Metal
Fe2+ forms pale green complexes.
Fe3+ forms yellow-brown complexes.
Ligand Type
Different ligands cause different amounts of d-orbital splitting, changing the light absorbed.
Example:
[Cr(OH)6]3− → Dark Green
[Cr(NH3)6]3+ → Purple
Complex Geometry
Octahedral complexes absorb different wavelengths than tetrahedral complexes.
How do transition metals act as catalysts?
Mechanisms:
Surface adsorption (heterogeneous catalysis).
Oxidation state changes (homogeneous catalysis).
Example: Iron (Fe) in the Haber Process → Switches between Fe2+ and Fe3+ to stabilize intermediates.
Real-World Applications of Colour in Transition Metals

What is a complex ion, and why do transition elements form them?
Definition: A central metal ion surrounded by ligands.
Why they form:
Different oxidation states allow bonding to a variety of ligands.
Examples:
Chromium(III) complexes:
[Cr(NH₃)₆]³⁺
[Cr(OH)₆]³⁻
[Cr(H₂O)₆]³⁺
Why do transition elements have variable oxidation states?
Cause: Similar energies of the 3d and 4s sub-shells allow electrons to be lost from both orbitals.
Key Features:
The 4s electrons are lost first, followed by some 3d electrons.
Since d-electrons are not completely shielded, they can be removed at different levels, creating multiple oxidation states.

Why do transition elements behave as catalysts?
Reasons:
Variable oxidation states → Allows them to accept and donate electrons, stabilizing reaction intermediates.
Vacant d orbitals → Can form dative bonds with reactants, lowering activation energy.

Why do transition elements form complex ions?
Cause: Vacant d orbitals are energetically accessible, allowing them to bond with ligands via dative covalent bonds.
Definition: A complex ion consists of a central metal ion surrounded by ligands.
Key Features:
Ligands donate lone pairs to form dative covalent bonds.
Complexes can have various coordination numbers (e.g., 6 for octahedral, 4 for tetrahedral).
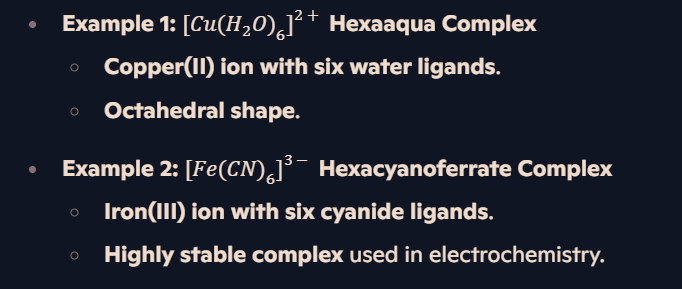
What is a ligand in a complex ion?
Definition: A species with a lone pair of electrons, which can form a dative covalent bond with a metal ion.
Types:
Neutral Ligands → H₂O, NH₃.
Negatively Charged Ligands → Cl⁻, OH⁻.
What is coordination number in complex ions?
Definition: The number of dative covalent bonds formed between the metal ion and ligands.

How do transition metals stabilize reaction intermediates?

What is the significance of vacant d orbitals in transition metal chemistry?
Vacant d orbitals allow complex formation by accepting electron pairs from ligands.
They enable catalysis by temporarily binding to reactants.
They contribute to variable oxidation states by participating in redox reactions.
What are common shapes of transition metal complexes?

What is a ligand?
Definition: A ligand is a molecule or ion with lone pairs of electrons, which forms dative covalent bonds with a central metal ion.
Key Features:
Electron donor → Donates a lone pair to the metal.
Forms coordinate bonds → Dative covalent bonding.

What is a complex ion?
Definition: A complex ion consists of a central metal ion surrounded by ligands.
Key Features:
Ligands form dative covalent bonds.
Overall charge depends on the metal ion and ligands.
What are monodentate ligands?
Can form only one dative bond.
Examples: H₂O, NH₃, Cl⁻, CN⁻
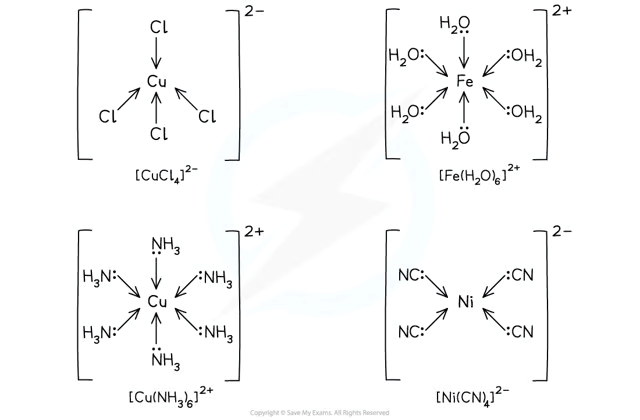
What are bidentate ligands?
Bidentate Ligands → Can form two dative bonds.
Examples: 1,2-diaminoethane ("en"), Ethanedioate ("ox").
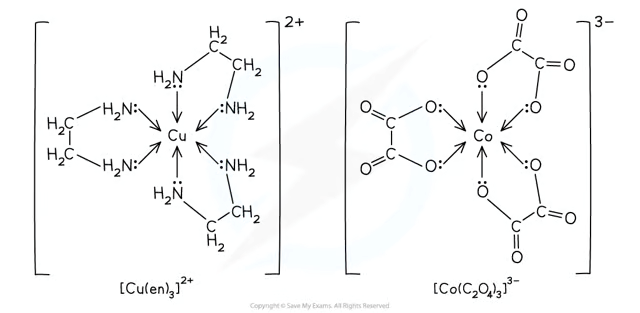
What are polydentate ligands
Polydentate Ligands → Can form multiple dative bonds.
Example: EDTA⁴⁻ → Hexadentate ligand.

How do transition metals react with ligands to form complexes?

What determines the shape of a complex ion?
Coordination Number (number of ligands bound to metal).
Common Shapes:
Octahedral (coordination number 6) → [Cu(H2O)6]2+.
Tetrahedral (coordination number 4) → [CoCl4]2−.
Square Planar (coordination number 4) → [Pt(NH3)2Cl2].
How does ligand size affect complex formation?

How do transition metal complexes change colour?

What is ligand exchange?

What are the common geometries of transition element complexes?

What is the linear geometry of transition element complexes?

What is the tetrahedral geometry of transition element complexes?

What is the square planar geometry of transition element complexes?

What is the octahedral geometry of transition element complexes?

What is coordination number?
Definition: The number of coordinate (dative covalent) bonds formed between the central metal ion and ligands.
It does not depend on the number of ligands, only on the number of bonds.

How do you predict the formula and charge of a complex ion?
Steps:
Identify central metal ion and its charge.
Identify ligands and their charges.
Determine coordination number (geometry).
Calculate total charge (sum of metal charge and ligand charges).

How does ligand size affect complex formation?

How do transition metals form complex ions?

What determines complex charge?
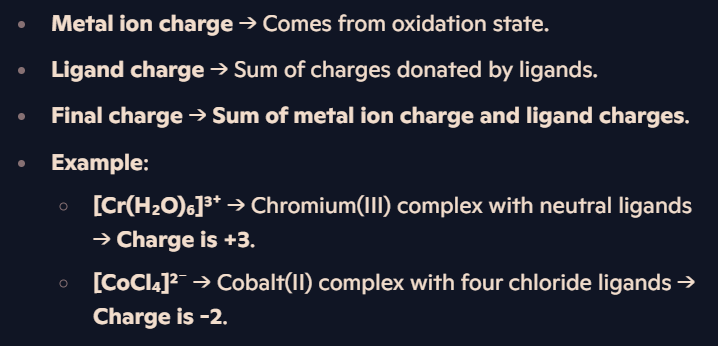
What is ligand exchange?
Definition: Ligand exchange (or ligand substitution) occurs when one ligand in a complex is replaced by another.
Key Features:
Can be partial or complete substitution of ligands.
Forms a more stable complex than the original.
No change in coordination number or geometry if the new ligand is of similar size.
Change in coordination number and geometry if the new ligand is larger or smaller than the original.
What is the ligand exchange reaction in copper(II) complexes?

What is the ligand exchange reaction in cobalt(II) complexes?
1. Hydroxide Ligand Exchange
Starting Complex:
[Co(H2O)6]2+(Pink solution)
Reaction with NaOH (partial substitution):
[Co(H2O)6]2++2OH−→[Co(H2O)4(OH)2](Blue precipitate)Excess alkali and warming → Red precipitate.
2. Ammonia Ligand Exchange
Reaction with excess NH₃ (complete substitution):
[Co(H2O)6]2++6NH3→[Co(NH3)6]2++6H2O
Colour change: Pink → Brown.
Further oxidation in air:
[Co(NH3)6]2+→[Co(NH3)6]3+
Final Colour: Dark brown (Cobalt(III) complex).
3. Chloride Ligand Exchange
Reaction with HCl (complete substitution):
[Co(H2O)6]2++4Cl−→[CoCl4]2−+6H2O
Colour change: Pink → Blue.
Coordination number change: 6 → 4 (octahedral → tetrahedral geometry).
Adding water reverses reaction, restoring pink colour.
How does ligand size affect ligand exchange?

Why is ligand exchange reversible?

What causes colour changes during ligand exchange?

How does coordination number change in ligand exchange?

How does ligand exchange affect stability?
Stronger ligand-metal interactions lead to more stable complexes.
Example:
NH₃ forms a more stable complex with Cu²⁺ than H₂O.
Cl⁻ forms a weaker complex, leading to reversible ligand exchange.
What is the overall impact of ligand exchange?
Changes in colour, shape, and coordination number.
Reversibility based on ligand interactions.
Important for biological and industrial chemistry (e.g., hemoglobin, catalysis).
What is a redox reaction, and how are transition metals involved?
Definition: A redox reaction is a reaction in which one species is oxidized (loses electrons) and another is reduced (gains electrons).
Role of Transition Metals:
Transition metals have variable oxidation states, enabling electron transfer in redox reactions.
Example:
Iron(III) ions can be reduced to Iron(II) ions.
Copper(I) ions can be oxidized to Copper(II) ions.
:What is the standard electrode potential (E∘), and how does it help predict reaction feasibility?
Definition: E∘ is the electrode potential measured under standard conditions relative to the standard hydrogen electrode (E∘ = 0 V).
Key Interpretations:
More positive E∘ → The species is more likely to gain electrons (be reduced).
More negative E∘ → The species is more likely to lose electrons (be oxidized).
How do you use E∘ values to determine if a reaction is feasible?

Example: Is the reduction of Fe³⁺ by Cu²⁺ feasible?

Why do some feasible reactions not occur spontaneously?
Standard electrode potentials only predict feasibility, not rate.
Some reactions have high activation energies, preventing spontaneous occurrence.
Example:
Conversion of Fe³⁺ to Fe²⁺ is thermodynamically feasible (E∘cell is positive).
But reaction may need a catalyst to lower activation energy.
How do transition metals change oxidation states in redox reactions?
Electrons are transferred, causing the metal ion to gain or lose oxidation state.
Example:
Iron changes between +2 and +3 oxidation states in different redox reactions.
Manganese changes between +2, +4, +6, and +7 oxidation states.
How do you balance redox reactions using half-equations?
Write each half-equation separately (one oxidation, one reduction).
Balance atoms (except H and O).
Balance oxygen using H₂O.
Balance hydrogen using H⁺.
Balance charge using electrons.
Multiply to ensure equal electron transfer, then combine.
Example: Redox titration involving MnO₄⁻ and Fe²⁺?

How does E° influence industrial processes?
Reactions with highly positive E° values are often used in electrochemical cells.
Feasible but slow redox reactions may require catalysts in industry.
Example:
Electrolysis reactions require external voltage to drive non-spontaneous redox changes.
How do transition metals act as redox catalysts?
They can switch oxidation states easily, stabilizing intermediate steps.
Example:
Manganese in MnO₄⁻ reduction can act as an autocatalyst, speeding up reactions as Mn²⁺ accumulates.
Importance in industry:
Used in catalytic converters, biochemical reactions, and industrial metal purification.
What is a redox reaction?
Definition: A reaction where one species loses electrons (oxidation) and another gains electrons (reduction).
Key Concept:
Oxidation → Increase in oxidation state.
Reduction → Decrease in oxidation state.
Example: Fe2+→Fe3++e−
(Iron(II) is oxidized to Iron(III), losing an electron.)
How do you construct a balanced redox equation?
Write separate half-equations for oxidation & reduction.
Balance atoms (except H & O).
Balance oxygen using H₂O.
Balance hydrogen using H⁺.
Balance charge using electrons.
Ensure equal electron transfer, then combine half-equations.
What is the redox reaction between MnO₄⁻ and C₂O₄²⁻ in acid?

What is autocatalysis in the MnO₄⁻ / C₂O₄²⁻ reaction?
Definition: A reaction catalyzed by one of its own products.
Mechanism:
Mn²⁺ ions formed accelerate the reaction.
The more Mn²⁺, the faster the reaction proceeds.
What is the redox reaction between MnO₄⁻ and Fe²⁺ in acid?
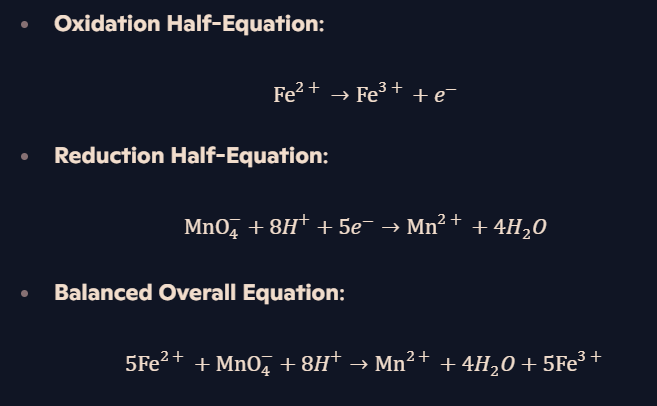
How does titration of MnO₄⁻ / Fe²⁺ work?
MnO₄⁻ is added dropwise into Fe²⁺ solution.
End-point: First trace of permanent pink appears.
Reason: MnO₄⁻ is slightly in excess and turns pink.
Used to determine Fe²⁺ concentration in unknown solutions.
What is the redox reaction between Cu²⁺ and I⁻?
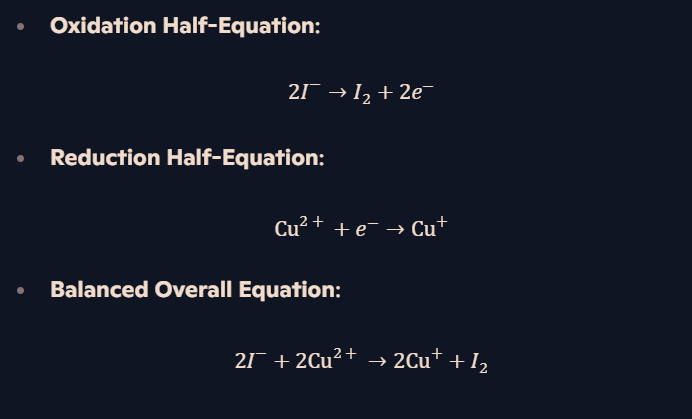
What happens when excess I⁻ is used with Cu²⁺?

How is Cu²⁺ concentration determined using thiosulfate titration?
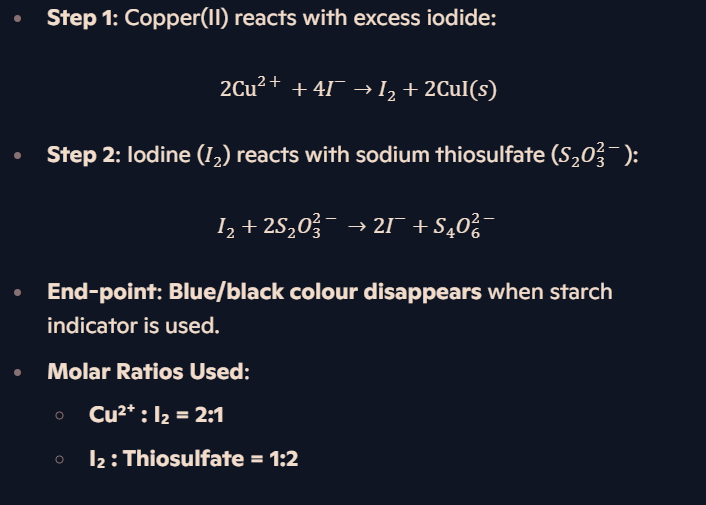
What are key redox calculations for transition metals?
Construct balanced redox equations.
Calculate oxidation states of metals.
Determine oxidizing & reducing agents.
Calculate standard electrode potentials (E∘).
Use molar ratios to find concentrations in titrations.
What are degenerate and non-degenerate d orbitals?
Degenerate Orbitals:
Definition: Five d orbitals in an isolated transition metal ion that have equal energy.
Example: 3d orbitals in a free metal ion.
Non-Degenerate Orbitals:
Definition: When ligands attach, the d orbitals split into two sets with different energy levels.
Cause: Dative bonding from ligands creates electrostatic repulsion, leading to orbital splitting.
How do degenerate d orbitals split upon ligand bonding?
Before bonding → All five d orbitals have equal energy (degenerate).
After bonding → Orbitals split into two sets (non-degenerate).
Splitting depends on complex geometry:
Octahedral Complexes → Two higher-energy orbitals, three lower-energy orbitals.
Tetrahedral Complexes → Three higher-energy orbitals, two lower-energy orbitals.
What happens in octahedral complexes?
Six ligands surround the metal ion.
Electrons in the dx²-y² and dz² orbitals experience more repulsion because these orbitals align with ligand approach.
Splitting pattern:
Higher-energy orbitals → dx²-y² and dz².
Lower-energy orbitals → dxy, dxz, dyz.
Energy difference labeled as ΔE.
What happens in tetrahedral complexes?
Four ligands surround the metal ion.
Electrons in the dxy, dxz, dyz orbitals experience more repulsion because these orbitals align with ligand approach.
Splitting pattern:
Higher-energy orbitals → dxy, dxz, dyz.
Lower-energy orbitals → dx²-y² and dz².
Energy difference labeled as ΔE.
Why does orbital splitting occur?
Ligands donate lone pairs, creating electrostatic repulsion.
Orbitals aligned with ligand approach experience greater repulsion, moving to higher energy levels.
Orbitals positioned between ligand approach experience less repulsion, moving to lower energy levels.
How does ΔE affect complex color?
ΔE determines the wavelength of light absorbed.
Electrons absorb energy to jump from lower to higher orbitals.
The color observed is the complementary color of absorbed light.
Example:
Large ΔE → Absorbs higher-energy light (violet/blue), appears yellow/orange.
Small ΔE → Absorbs lower-energy light (red), appears green/blue.
How does complex geometry affect ΔE?
Octahedral complexes → Larger ΔE due to stronger ligand repulsion.
Tetrahedral complexes → Smaller ΔE due to weaker ligand repulsion.
ΔE influences absorption of visible light, affecting complex color.
What are real-world applications of d orbital splitting?
Transition metal complexes in dyes and pigments.
Biological systems (e.g., hemoglobin, chlorophyll).
Catalysis in industrial processes (e.g., hydrogenation, oxidation reactions).
Why do transition element complexes appear coloured, and how does electron promotion explain this phenomenon?
Electron Promotion:
Definition: When light shines on a transition element complex, an electron absorbs a precise amount of energy (ΔE) and gets promoted from a lower non-degenerate d orbital to a higher non-degenerate d orbital.
Cause: Ligands split the originally degenerate d orbitals, creating two non-degenerate energy levels.
Colour Formation:
Light Absorption: The complex absorbs specific frequencies of light from the visible spectrum.
Complementary Colour: The frequencies not absorbed combine to create the colour observed.
Example: Copper(II) ions absorb red light, leading to a pale blue (cyan) appearance.
How does the type of ligand influence the colour of a transition element complex?
Ligand Effect on ΔE:
Definition: Different ligands split the d orbitals by different amounts of energy due to varying levels of repulsion, modifying ΔE.
Result: This changes the frequency of light absorbed, altering the observed complementary colour.
Examples:
[Cu(H₂O)₆]²⁺ → Light blue (Water ligands cause smaller ΔE)
[Cu(NH₃)₄(H₂O)₂]²⁺ → Dark blue (Ammonia ligands cause greater ΔE)
Despite both complexes containing Cu²⁺, their ligands influence orbital splitting, affecting the observed colour.
What happens to the colour of transition metal complexes when ligand exchange occurs in Cu²⁺ and Co²⁺ complexes?
Ligand Exchange Effect:
Definition: Changing ligands modifies d orbital splitting, altering ΔE and shifting the absorbed light frequency.
Result: The observed colour changes due to a different complementary colour being formed.
Examples:
Ammonia Ligand Exchange:
[Cu(H₂O)₆]²⁺ (light blue) → [Cu(NH₃)₄(H₂O)₂]²⁺ (deep blue)
[Co(H₂O)₆]²⁺ (pink) → [Co(NH₃)₆]²⁺ (brown)
Chloride Ligand Exchange:
[Cu(H₂O)₄(OH)₂] (pale blue precipitate) → [CuCl₄]²⁻ (yellow solution)
[Co(H₂O)₄(OH)₂] (blue precipitate) → [CoCl₄]²⁻ (blue solution)
Ligand exchange alters ΔE, shifting the absorbed frequency and changing the observed colour.
How does the equation ΔE = h × v explain the relationship between light absorption and colour changes in transition element complexes?
Mathematical Relationship:
Equation: ΔE = h × v
h = Planck’s constant (6.626 × 10⁻³⁴ m² kg s⁻¹)
v = frequency of absorbed light (Hz)
Light Absorption: Electrons absorb exactly ΔE, promoting them between non-degenerate d orbitals.
Effect on Colour:
The size of ΔE depends on ligand type.
Different ligands affect ΔE, changing the frequency of absorbed light.
This determines the complementary colour observed in solution.
What types of stereoisomerism do transition element complexes exhibit, and how do geometrical and optical isomers form?
Stereoisomerism in Complexes: Transition metal complexes can show geometrical (cis/trans) isomerism and optical isomerism due to different ligand arrangements.
Geometrical (Cis/Trans) Isomerism:
Occurs in square planar and octahedral complexes with two different pairs of ligands.
Square Planar Example:
Cisplatin ([Pt(NH₃)₂Cl₂]) is used in cancer treatment because it can bind to DNA.
Transplatin has chloride ligands opposite each other and lacks medical benefits.
Octahedral Example:
[Co(NH₃)₄(H₂O)₂]²⁺ and [Ni(en)₂(H₂O)₂]²⁺ exhibit cis-trans isomerism.
Cis-isomer: Two different ligands are next to each other.
Trans-isomer: Two different ligands are opposite each other.
Optical Isomerism:
Occurs in octahedral complexes with bidentate ligands like [Ni(en)₃]²⁺ and [Ni(en)₂(H₂O)₂]²⁺.
Optical isomers are non-superimposable mirror images that rotate plane-polarized light differently.
They lack a plane of symmetry, making them truly distinct.
How does geometrical isomerism affect the polarity of transition element complexes?
Polarity in Square Planar Complexes:
Cisplatin ([Pt(NH₃)₂Cl₂]) has both chlorine ligands on the same side, causing charge imbalance, making it polar.
Transplatin has chlorine ligands opposite each other, balancing the charge, making it non-polar.
Polarity in Octahedral Complexes:
Cis-[Co(NH₃)₄(H₂O)₂]²⁺ is polar because water ligands cause charge imbalance (oxygen is more electronegative than nitrogen).
Trans-[Co(NH₃)₄(H₂O)₂]²⁺ is non-polar due to symmetrical ligand arrangement, balancing charge distribution.
What is the stability constant (Kstab), and how does it relate to ligand exchange in transition element complexes?
Definition:
Kstab is the equilibrium constant for the formation of a complex ion in a solvent from its constituent ions or molecules.
It indicates how stable a complex is compared to its free metal ion and ligands.
Formation of Complex Ions:
Transition element ions in aqueous solutions become hydrated, forming complexes like [Co(H₂O)₆]²⁺.
When other ligands are present, ligand exchange occurs, leading to a more stable complex.
Example: Adding ammonia stepwise to [Cu(H₂O)₆]²⁺ leads to [Cu(NH₃)₄(H₂O)₂]²⁺, a more stable complex.
Kstab & Complex Stability:
Larger Kstab → More stable complex (favored equilibrium).
Smaller Kstab → Less stable complex (less favored equilibrium).
How is the expression for Kstab written, and why is [H₂O] excluded?
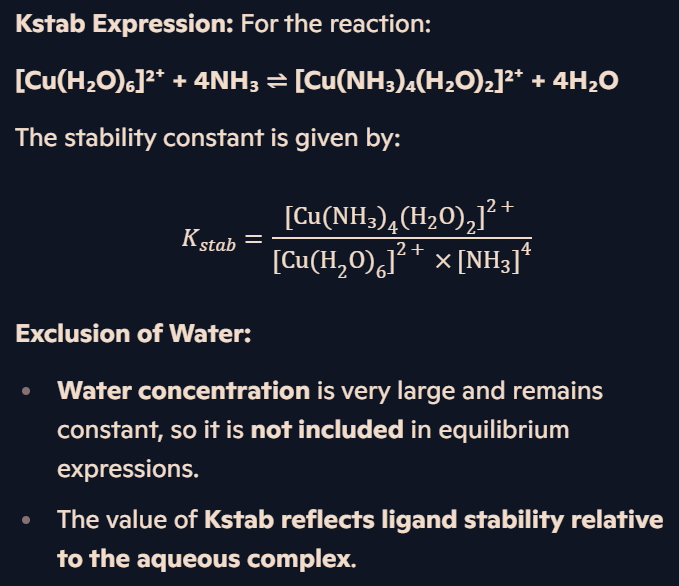
How can Kstab expressions be used to compare complex stability and determine equilibrium positions?
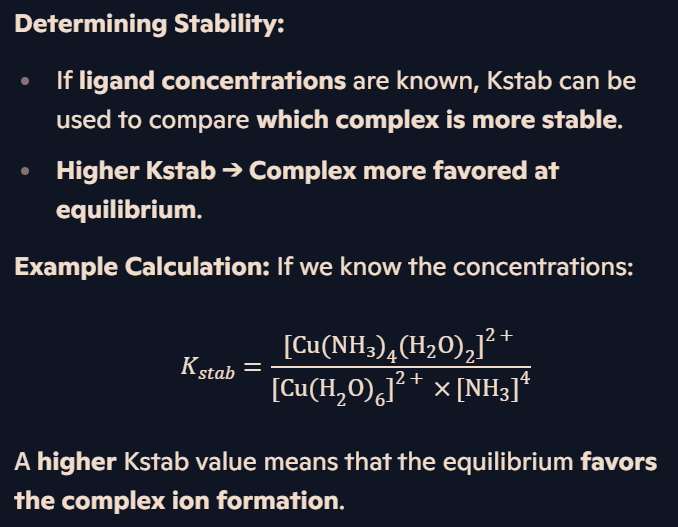
How does ligand exchange affect Kstab, and why does a larger Kstab indicate a more stable complex ion?
Ligand Exchange:
Ligand substitution occurs to form a more stable complex with a larger Kstab.
Stability constants are often expressed on a log₁₀ scale for easier comparison.
Example of Ligand Substitution: When ammonia is added to [CoCl₄]²⁻, the chloride ligands are replaced, forming a more stable ammonia complex.
![<p><strong>Ligand Exchange:</strong></p><ul><li><p>Ligand substitution occurs to form a <strong>more stable complex</strong> with a <strong>larger Kstab</strong>.</p></li><li><p>Stability constants are often expressed on a <strong>log₁₀ scale</strong> for easier comparison.</p></li></ul><p><strong>Example of Ligand Substitution:</strong> When ammonia is added to <strong>[CoCl₄]²⁻</strong>, the chloride ligands are replaced, forming a more stable <strong>ammonia complex</strong>.</p>](https://knowt-user-attachments.s3.amazonaws.com/2be38946-41db-4371-983b-8cd036852154.png)
What is the trend in the thermal stability of Group 2 nitrates and carbonates?
Definition: Thermal stability refers to the resistance of a compound to decomposition when heated.
Trend: Thermal stability increases down Group 2.
Reason:
Smaller metal cations (top of Group 2) have higher charge density, causing greater polarisation of the nitrate (NO3−) and carbonate (CO32−) anions.
Polarisation weakens bonds, requiring less heat to decompose.
Larger metal cations (bottom of Group 2) have lower charge density, less polarising effect, and thus higher thermal stability.
How does ionic radius affect the polarisation of the nitrate and carbonate anions?
Smaller cations (e.g., Be2+, Mg2+):
Higher charge density → Strong attraction to anion → Greater distortion of electron cloud → Weaker bonds → Lower thermal stability.
Larger cations (e.g., Ba2+):
Lower charge density → Weaker attraction to anion → Less distortion → Stronger bonds → Higher thermal stability.
Example:
MgCO₃ decomposes more easily than BaCO₃ due to greater polarisation.
What are the thermal decomposition reactions of Group 2 nitrates and carbonates?

What is the solubility trend of Group 2 hydroxides?
Trend: Solubility increases down the group.
Example: Mg(OH)₂ is less soluble than Ba(OH)₂.
Reason:
Smaller cations (Mg2+) form stronger lattice interactions, requiring more energy to break apart → Lower solubility.
Larger cations (Ba2+) form weaker lattice interactions, making them easier to dissolve → Higher solubility.
What is the solubility trend of Group 2 sulfates?
Trend: Solubility decreases down the group.
Example: BaSO₄ is nearly insoluble in water, whereas MgSO₄ is quite soluble.
Reason:
Large sulfate anion (SO42-) forms strong lattice interactions with larger cations, making it harder to dissolve.
Down the group, lattice energy does not decrease as fast as hydration energy, making solubility lower.
What is enthalpy change of solution (ΔH∘sol)?

What is lattice energy (ΔH∘latt)?
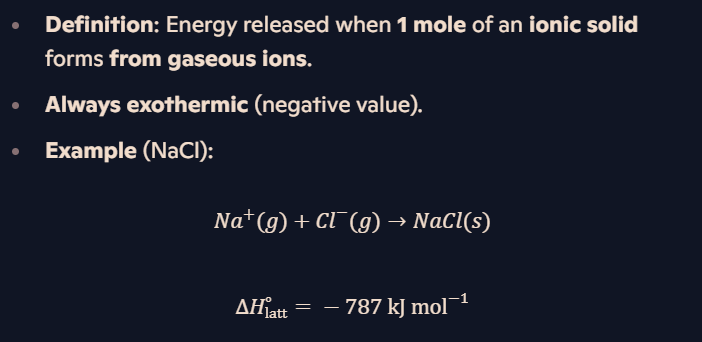
What is hydration enthalpy (ΔH∘hyd)?
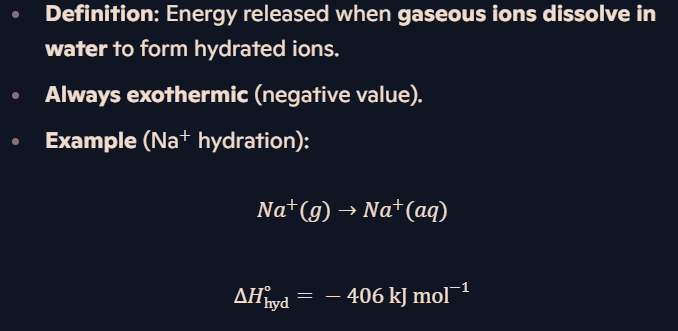
What is the trend in enthalpy change of solution (ΔH∘sol) down Group 2?
Group 2 hydroxides → More exothermic, increasing solubility.
Group 2 sulfates → More endothermic, decreasing solubility.
Reason:
Hydration energy decreases down the group (weaker ion-dipole bonds).
Lattice energy decreases down the group, but not as rapidly for sulfates.
Why are sulfates less soluble but hydroxides more soluble down Group 2?
Hydroxides:
OH- is small, so lattice energy drops faster than hydration enthalpy → More exothermic ΔH∘sol → Higher solubility.
Sulfates:
SO42− is large, so lattice energy stays strong while hydration enthalpy drops → More endothermic ΔH∘sol→ Lower solubility.
What is Thin Layer Chromatography (TLC), and what are its phases?
Definition:
TLC is a technique used to separate and analyze small samples of mixtures.
Example: Separating dyes in forensic samples.
Phases in TLC:
Stationary Phase:
A thin layer of alumina (Al₂O₃) or silica (SiO₂) coated on a solid support (e.g., metal sheet).
Solute molecules adsorb onto the surface.
Components interact with the stationary phase based on polarity and adsorption strength.
Mobile Phase:
A polar or non-polar solvent or gas that flows over the stationary phase.
Carries the components of the sample being analyzed.
Examples:
Polar solvents: Water, alcohols.
Non-polar solvents: Alkanes.
Key Principle:
The separation depends on:
Interaction with the stationary phase: Stronger interactions cause components to "stick" and move slower.
Solubility in the mobile phase: Higher solubility allows components to travel further.
How is a TLC analysis conducted step-by-step?
Step-by-Step Procedure:
Prepare the Solvent:
Add a small quantity of solvent to a beaker.
Draw the Baseline:
Use a pencil to draw a horizontal line near the bottom edge of the TLC plate.
Why pencil? Ink would interact with the sample and solvents.
Spot the Samples:
Place a spot of pure reference compound on the left of the baseline.
Place a spot of the sample to be analyzed on the right.
Allow the spots to air dry.
Place the Plate in the Beaker:
Ensure the baseline is above the solvent level.
Cover the beaker with a lid.
Allow the Solvent to Travel:
The solvent moves up the plate, dissolving and carrying the sample components.
Mark the Solvent Front:
When the solvent reaches the top, remove the plate and draw a pencil line at the solvent's highest point (solvent front).
Result:
The sample components separate and travel different distances based on their interactions with the stationary phase and solubility in the mobile phase.
What are Rf values, and how are they calculated?

How can Rf values be interpreted?
Higher Rf Values:
Components travel further up the TLC plate.
Indicates:
Less polar components.
Higher solubility in the mobile phase.
Lower Rf Values:
Components stay closer to the baseline.
Indicates:
More polar components.
Stronger interaction with the stationary phase.
Practical Use:
Compare Rf values of unknown components with reference compounds to identify them.
Example: If an unknown component has an Rf value of 0.5 and matches the Rf value of a known compound, they are likely the same.
What causes differences in Rf values?
Factors Affecting Rf Values:
Interaction with the Stationary Phase:
Polar components interact strongly with polar stationary phases (e.g., silica or alumina).
Stronger interaction causes components to "stick" and move slower, resulting in lower Rf values.
Solubility in the Mobile Phase:
Non-polar components dissolve better in non-polar solvents (mobile phase).
Higher solubility allows components to travel further, resulting in higher Rf values.
Example:
Using a polar stationary phase (silica) and a non-polar solvent (hexane):
Non-polar component: High Rf value (travels further).
Polar component: Low Rf value (travels less).
What are the key terms and concepts in TLC?
Key Concepts:
Separation depends on:
Interaction with the stationary phase: Stronger interaction → Lower Rf value.
Solubility in the mobile phase: Higher solubility → Higher Rf value.
