Chemistry Regents Review: Acids, Bases, Salts & Unit 4.4 (week #8)
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Electrolyte
a substance that breaks up into charged ions when dissolved in water
Electrolytes will conduct an….
electrical current
higher concentration = more…
electricity
3 types of electrolytes
acids
bases
salts (ionic)
Salts
ionic compound and ionize in a solution
Arrhenius Acid
dissolve (produce) into H+ (hydrogen) or H3O+ (hydronium) as positive ions in solutions
Arrhenius Bases
dissolve (produce) OH- (hydroxide) as a negative ion
Brownsted-Lowry Acid
donates H+ ion
Brownsted Lowry Base
accepts H+ ion
neutral solution (pH 7)
contains hydrogen ions & hydroxide ions in = concentrations
pH
the measure of acidity of a substance or solution
Acidic
(H+ > OH-)
Basic
(H+ < OH-)
to differentiate 2 substances, use an….
indicator that would make them 2 different colors
For pH to lower by 1, there must be a….
tenfold (10x) increases in hydronium (H+) gas
For pH to raise by 1, there must be a…
tenfold decrease in hydroxide (OH-) ions
Neutralization
to make a solution neutral
acid + base → salt + water
Titration
solution of known concentration used to determine concentration of another
Hydrogen ions is referered to as a ________ b/c ion loses 1 e-
proton
Bronstead-Lowry Acid/Base Example
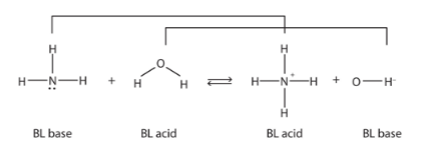
Conjugate Acid & Base Pairs
when an acid and base are related by the lose or gain of a single H+
Amphoteric
substance that can act as both an acid and a base
Amphoteric Example
H2O