Carboxylic Acids and their Derivatives: General Exam Questions
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
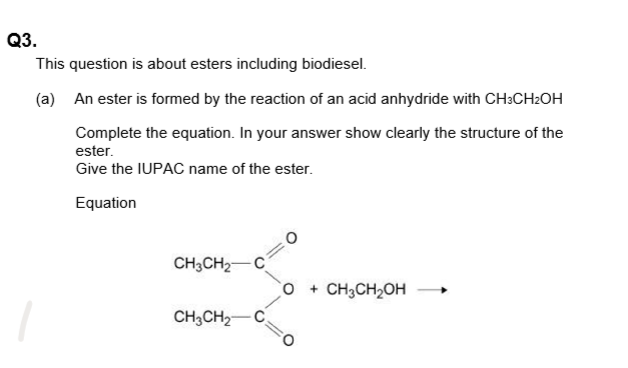
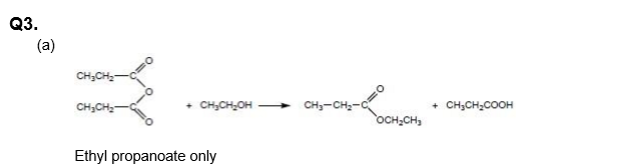
- Acid Anhydride + Alcohol → Ester + Carboxylic Acid

Triglyceride + 3NaOH → Glycerol + 3 Sodium Carboxylate
For saponification questions, remember it always reacts with 3NaOH,Glycerol stays the exact same, and the Carboxylate ions are of the longest of each chain (e.g.C17H33) but with an addition COONa
Biodiesels are the same but the Na switched for CH3
(methyl ester)
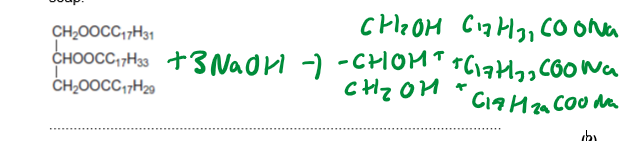
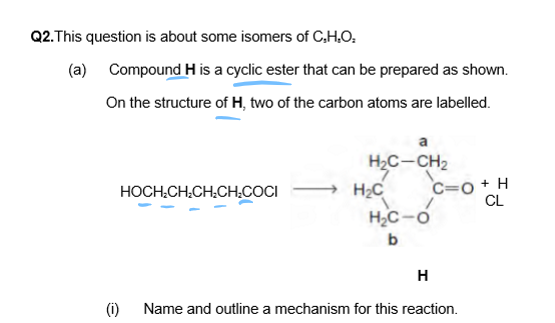
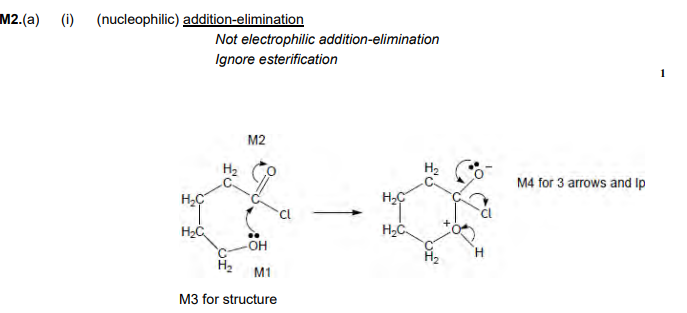
Nucleophilic Addition-Elimination
Cyclic compounds with OH react with themselves to form the compound, as shown here
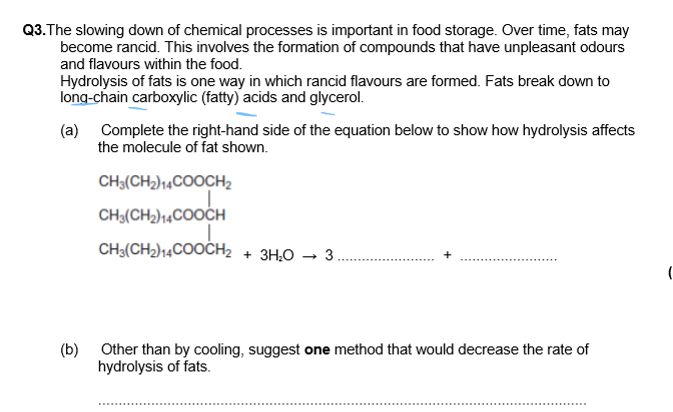
Ester + H₂O → Fatty Acid(Carboxylic Acid,RCOOH) + Glycerol(CH₂OHCHOHCH₂OH)
b)Keeping the foodstuff dry


Anti-oxidants react with the free radicals, and are used up in the reaction, hence not being regenerated.
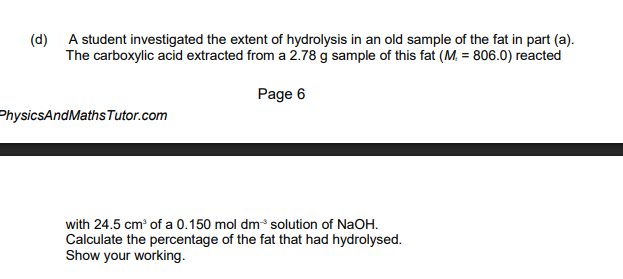
Titration Question
-Write out Equation in regards to NaOH, if not, usually a 1:1 MR
-Work backwards, always use ALL data provided
