Chapter 11 - Chemical Equilibrium
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
What occurs during dynamic equilibrium?
Rate of forward reaction will occur at the same rate as that of the reverse reaction
Once equilibrium is reached, do the forward and reverse reactions stop?
No, it is still ongoing such that the rate of production formation is equal to the rate of reactant formation
Do concentrations of reactants and products have to be equal at the time of equilibrium?
Not necessarily, but they can be
What is the law of mass action?
the rate of forward and reverse reaction is proportional to the product of concentrations of the reactants and products
What is the equilibrium constant (Keq)?
allow us to quantify the concentrations of reactants and products for a system in equilibrium under constant temperature
What is the formula for equilibrium constant given this formula, aA + bB ⟷ cC + dD?
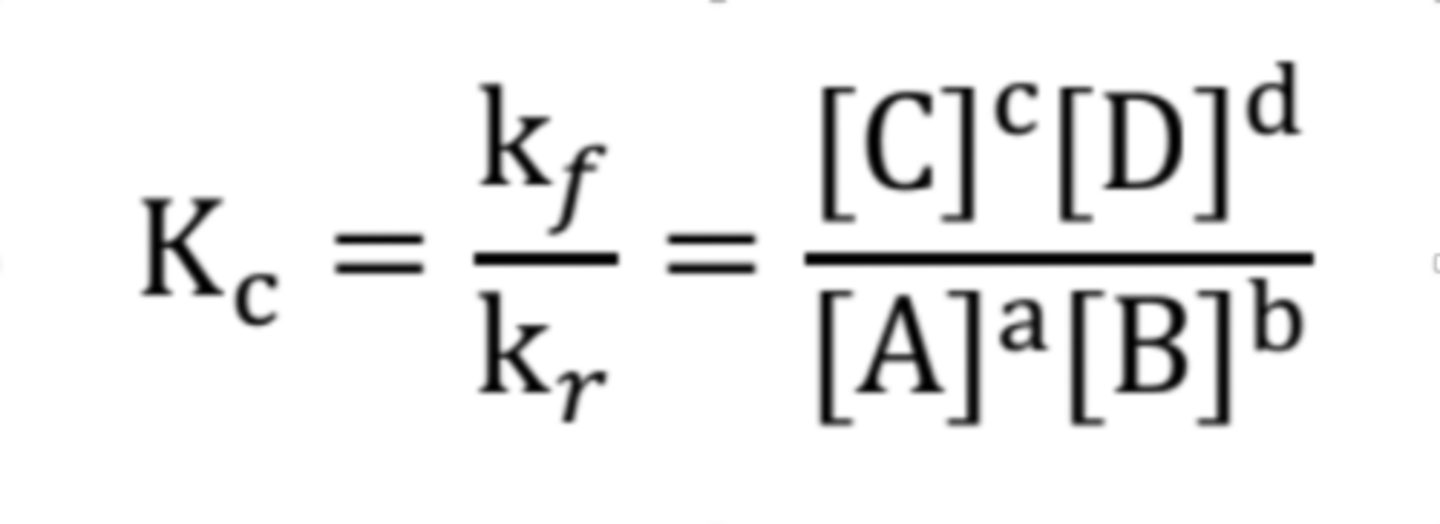
What does the Kc (concentrations) represents?
the equilibrium constant of aqueous or gaseous systems, expressed in concentrations of molarity
What does the Kp (pressures) represents?
the equilibrium constant for gaseous systems, expressed in partial pressures.
What is the Gibbs' free energy formula with the inclusion of equilibrium constant?
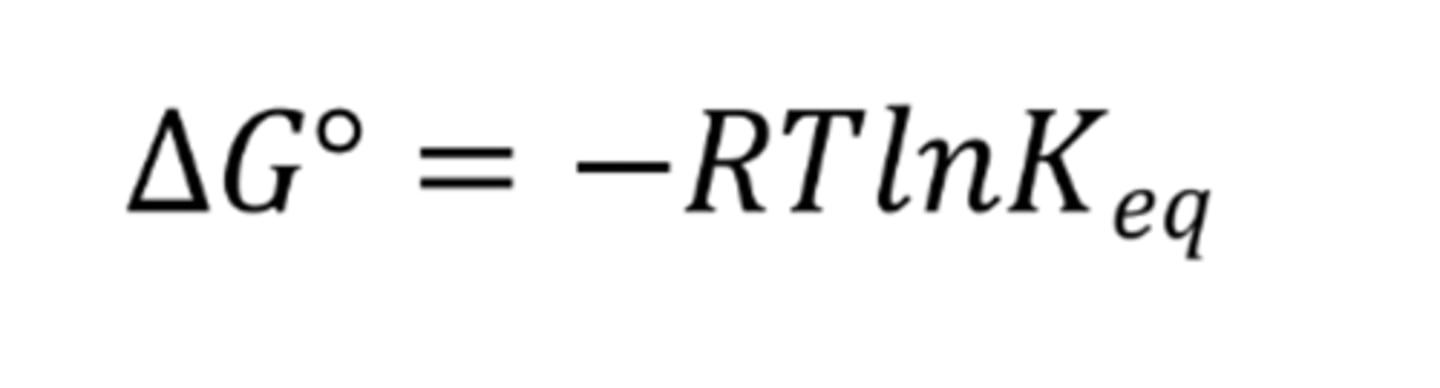
A Keq < 1 suggests what about the ratio of reactants and products at equilibrium?
Greater concentration of reactants than products at equilibrium
A Keq = 1 suggests what about the ratio of reactants and products at equilibrium?
Ratio of products to reactants at equilibrium is equal
A Keq > 1 suggests what about the ratio of reactants and products at equilibrium?
Greater concentration of products than reactants at equilibrium.
When Keq < 1, is ΔG° positive or negative?
When Keq < 1, ΔG° is positive and so the reactants are lower in energy and more stable than the products (there are more reactants)
When Keq = 1, is ΔG° positive or negative?
When Keq = 1, ΔG° is zero and so the ratio of products to reactants is equal
When Keq > 1, is ΔG° positive or negative?
When Keq > 1, ΔG° is negative and so the products are lower in energy and more stable than the reactants (there are more products)
What is the reaction quotient (Q)?
allow us to quantify the concentrations of reactant and products at any point of the reaction
What is different between reaction quotient (Q) and the equilibrium constant (K)?
Equilibrium constant is based off of concentrations once reaction has reached equilibrium. Reaction quotient uses concentrations at any point in time during a reaction other than equilibrium
If Q < Keq, what does this suggest about the concentration of reactants and products?
There is a higher concentration of reactants than there would be at equilibrium, as such the system shifts more towards the forward reaction to reach equilibrium
If Q = Keq, what does this suggest about the reaction?
The reaction is in dynamic equilibrium
If Q > Keq, what does this suggest about the reaction?
There is a higher concentration of products than there there would be at equilibrium, as such the system more towards the reverse reaction to reach equilibrium
What type of reactants and products are excluded from calculating equilibrium constant?
Liquid and solid state reactants or products
What does Le Châtelier's principle describe?
A system will shift in a direction that restores the equilibrium state in the presence of changes in concentration or in the temperature of the system
If reactants are added, what direction would the reaction shift?
Reaction would shift towards the forward reaction until Q = Keq again
If reactants are added, what does this mean for the reaction?
there is an increase in concentration of reactants, Q < Keq
If reactant is removed, what does this mean for the reaction?
there is a decrease in concentration of reactants, Q > Keq
If reactants are removed, what direction would the reaction shift?
Reaction would shift towards the reverse reaction until Q = Keq again
If products are added, what does this mean for the reaction?
there is an increase in concentration of products, Q > Keq
If products are added, what direction would the reaction shift?
Reaction would shift towards the reverse rearction until Q = Keq again
If products are removed, what does this mean for the reaction?
there is a decrease in concentration of products, Q < Keq
If products are removed, what direction would the reaction shift?
Reaction would shift towards the forward reaction until Q = Keq again.
If pressure is added to a system resulting in a decrease in volume, how would the equilibrium shift?
The equilibrium will shift towards the side of the reaction with a lower total number of moles of gas
If pressure is removed from a system resulting in an increase in volume, how would the equilibrium shift?
The equilibrium will shift towards the side of the reaction with a greater total number of moles of gas
If a reaction is endothermic in the forward direction, would heat be considered a reactant or a product?
Reactant
If a reaction is exothermic in the forward direction, would heat be considered a reactant or a product?
Product
If you lower the temperature in an endothermic forward reaction, what direction would the system shift?
Reaction will shift left as there is a decrease in heat
If you increase the temperature in an endothermic forward reaction, what direction would the system shift?
Reaction will shift right to absorb excess heat
If you increase the temperature in an exothermic forward reaction, what direction would the system shift?
Reaction will shift left as there is now more heat
If you decrease the temperature in an exothermic forward reaction, what direction would the system shift?
Reaction will shift right to produce more heat
What is the solubility product constant, Ksp, used to figure out?
Used to figure out direction in which reaction will proceed, but also the saturation of the solution and whether or not precipitation will occur
What is a precipitation reaction?
when ions of a solution react together to form an insoluble ionic solid, known as precipitate
When evaluating the Ksp of a susbtance, what do we need to remember?
Higher Ksp = more soluble
Lower Ksp = less soluble
What is the key difference between Ksp and Qsp?
Ksp tells us the solubility of a substance and is derived by using the concentration of ions for a saturated solution.
Qsp tells us the current state of the solution and is derived by using the concentration of ions for an unsaturated or supersaturated solution.
What relationship between Q and Ksp would suggest a saturated reaction mixture?
Q = Ksp
What relationship between Q and Ksp would suggest a supersaturated reaction mixture?
Q > Ksp
What relationship between Q and Ksp would suggest an unsaturated reaction mixture?
Q < Ksp
What relationship between Q and Ksp would suggest precipitation of a solute will occur?
Q > Ksp. Reaction is currently supersaturated and will proceed in reverse reaction to result in precipitation
What is the common-ion effect?
occurs when the presence of a common ion in a solution reduces the ionization/solubility of an ionic precipitate
What does the term amphoteric mean?
A substance that can act as both an acid and a base, depending on what it is reacting with
What is the autoionization of water?
the amphoteric property of H2O allows water molecules to react with other water molecules
What is the water dissociation constant (Kw)?
the equilibrium of the water autoionization reaction. Kw = 1×10-14
What is the general formula for the acidic dissociation constant, Ka?
Ka = [H+][A-]/[HA]
What is the general formula for the basic dissociation constant, Kb?
Kb = [OH-][HB+]/B]
How would the following reaction shift if HCl is added? HA (aq) + H2O (l) ⇄ H3O+ (aq) + A- (aq)
HCl is a strong acid and dissociates completely into H3O+. As such, more products than reactants so the reaction will shift left.
How would the following reaction shift if NaOH is added? HA (aq) + H2O (l) ⇄ H3O+ (aq) + A- (aq)
NaOH is a strong base and would immediately react with H3O+ and reduce the concentration. As such, the reaction will shift right
How would the following reaction shift if HA is added? HA (aq) + H2O (l) ⇄ H3O+ (aq) + A- (aq)
With more HA, there is more reactants than products. Reaction will shift right
How would the following reaction shift if A- is added? HA (aq) + H2O (l) ⇄ H3O+ (aq) + A- (aq)
With more A-, there is more products than reactants. Reaction will shift left
How would the following reaction shift if A- is removed? HA (aq) + H2O (l) ⇄ H3O+ (aq) + A- (aq)
Removing A- decreases the concentration of products, Reaction will shift right
How would the following reaction shift if the pH is increased? HA (aq) + H2O (l) ⇄ H3O+ (aq) + A- (aq)
Increasing pH reduces the concentration of H3O+.
Reaction will shift right