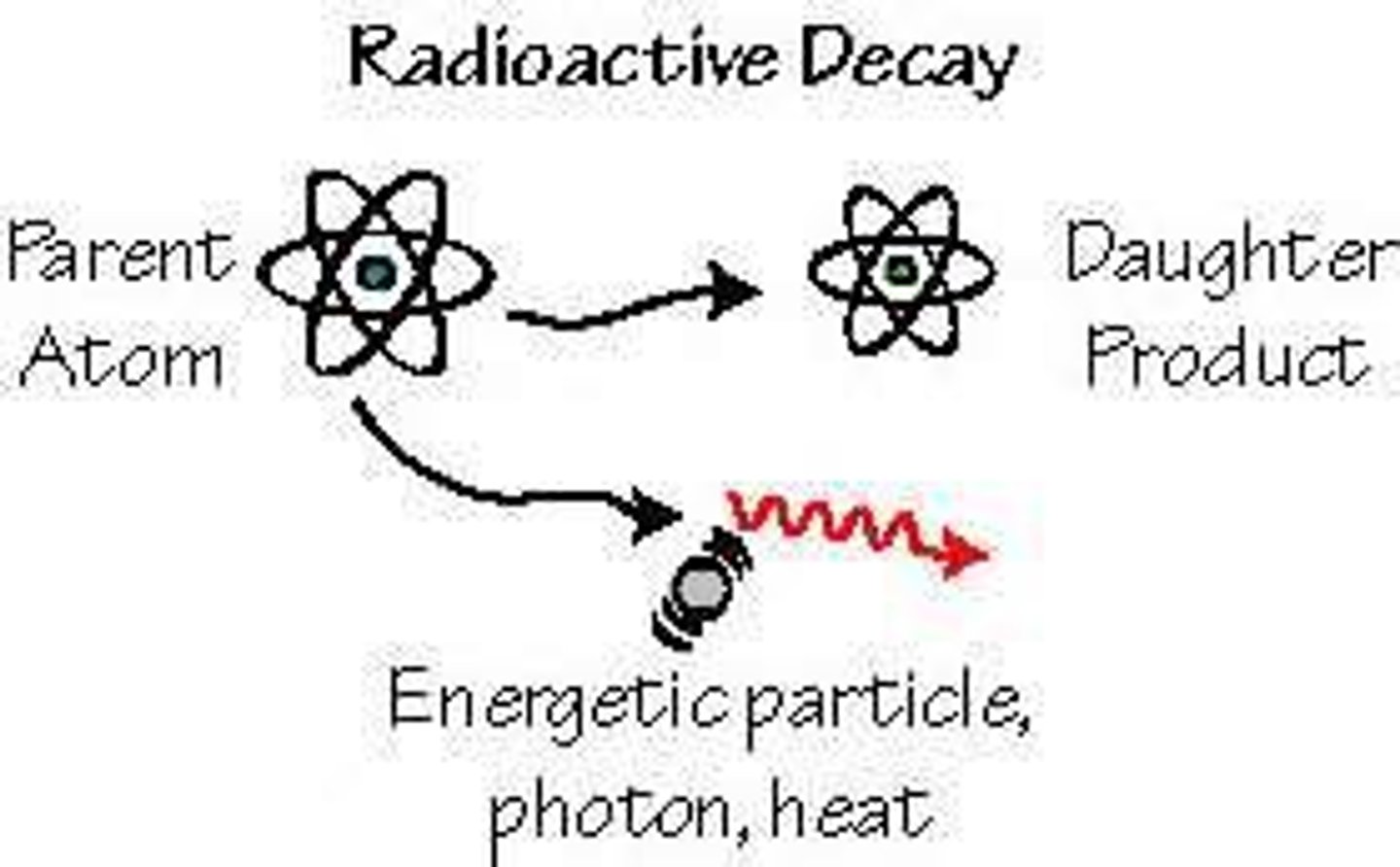
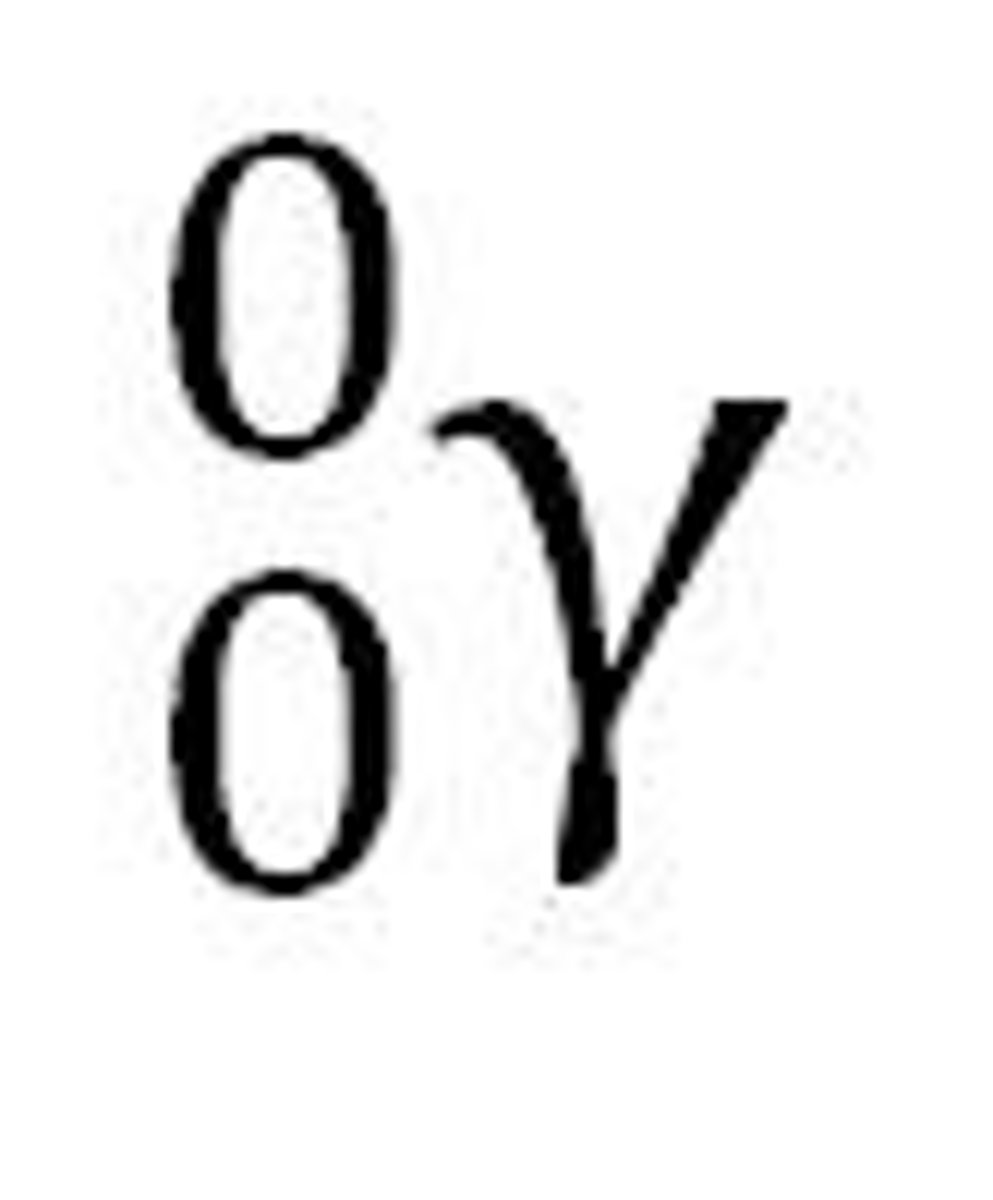Radioactive Decay
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
⁴₂He
A helium nucleus
beta particle
the particle formed by changing a neutron to a proton
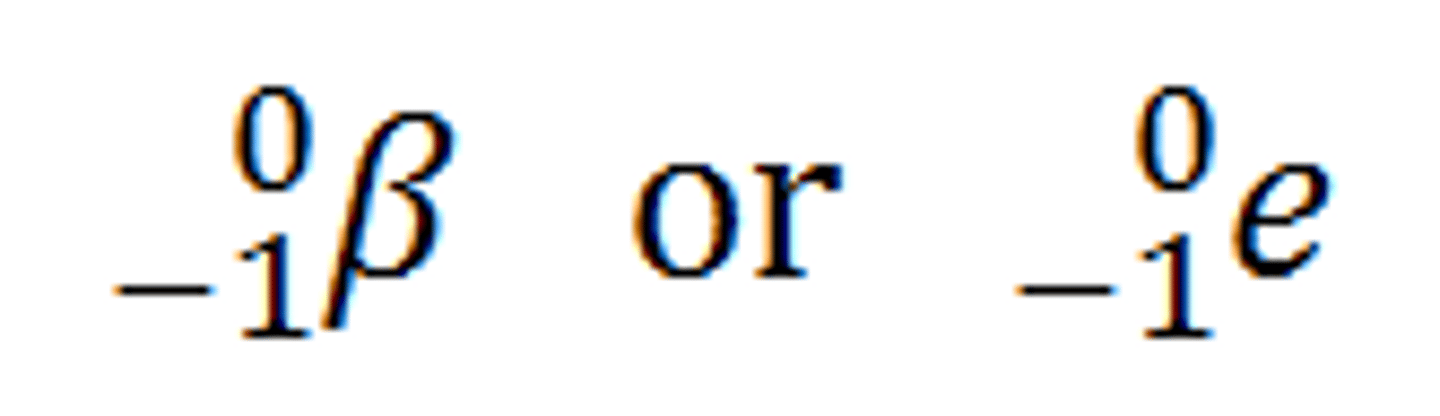
gamma rays
high energy rays & a type of electromagnetic radiation
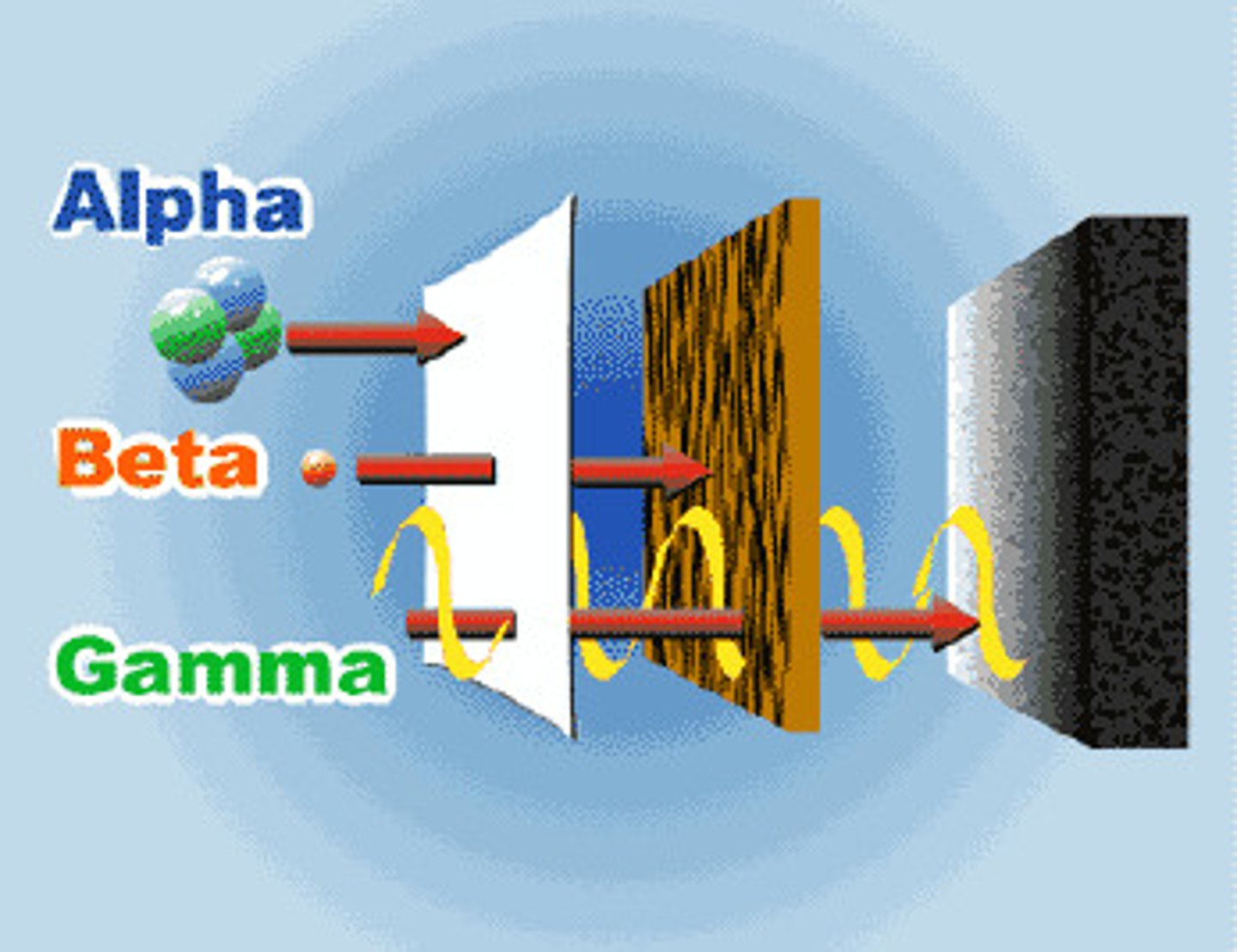
Alpha Decay
Mass number decreases by 4 (loss of 2 protons and 2 neutrons); Atomic number decreases by 2 - emits a helium nucleus
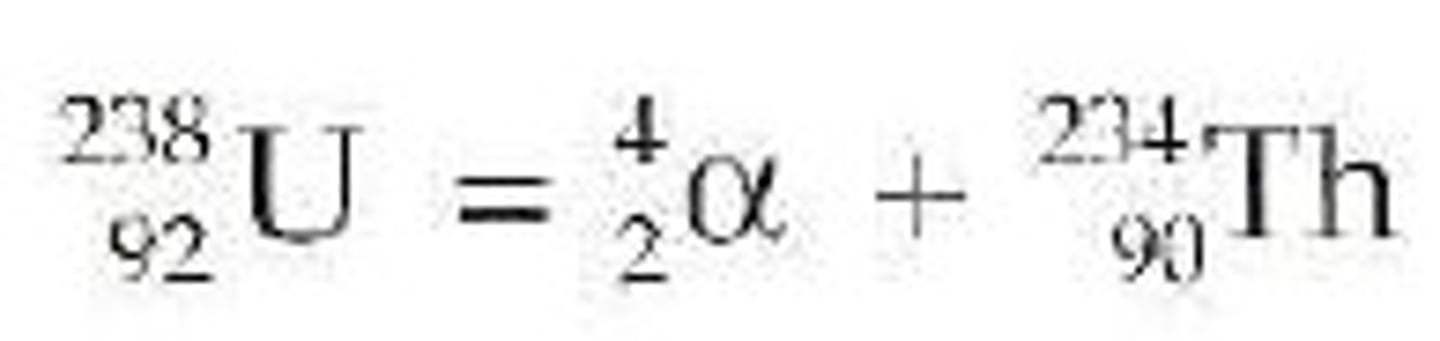
alpha particle
a positively charged particle consisting of two protons and two neutrons
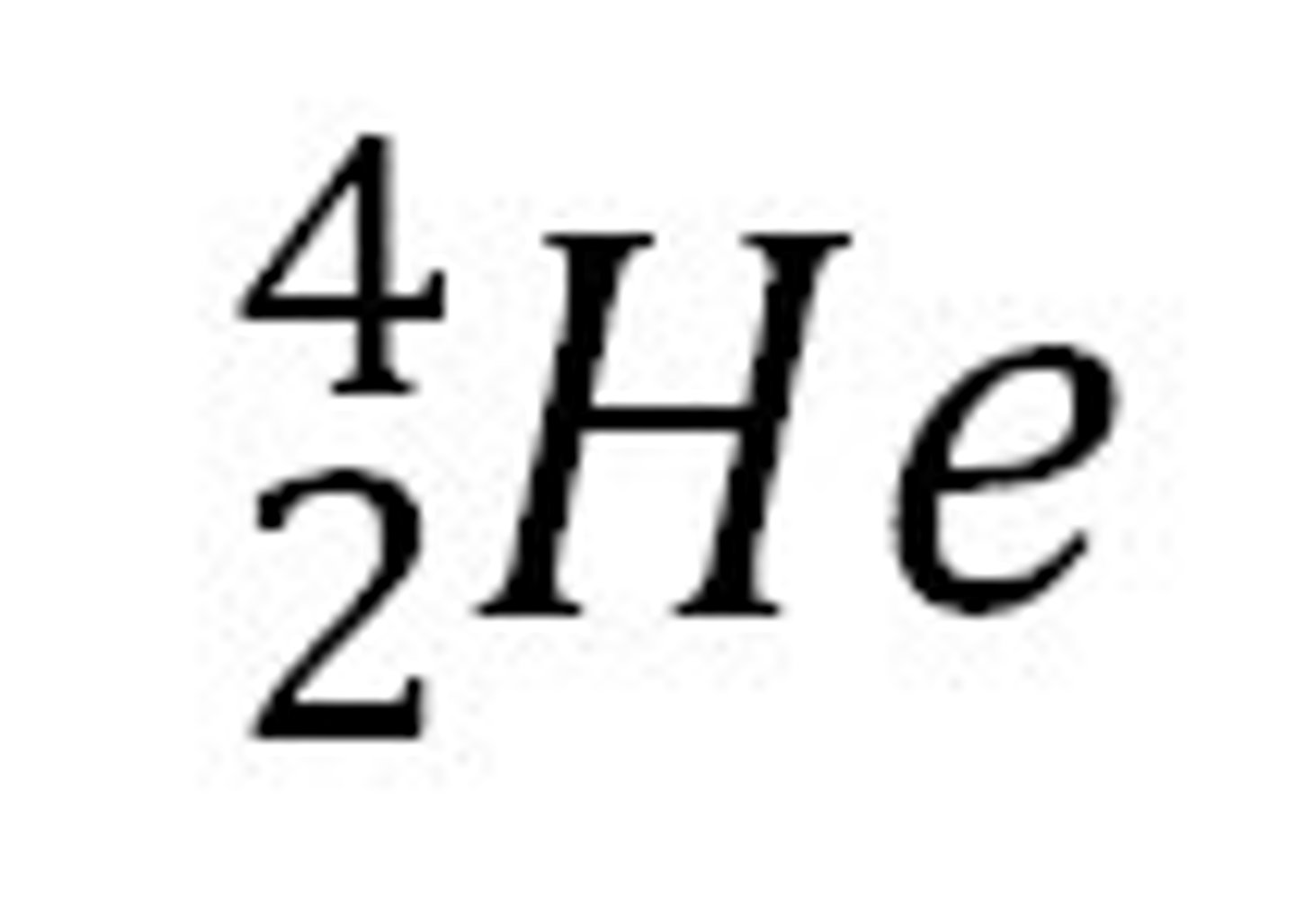
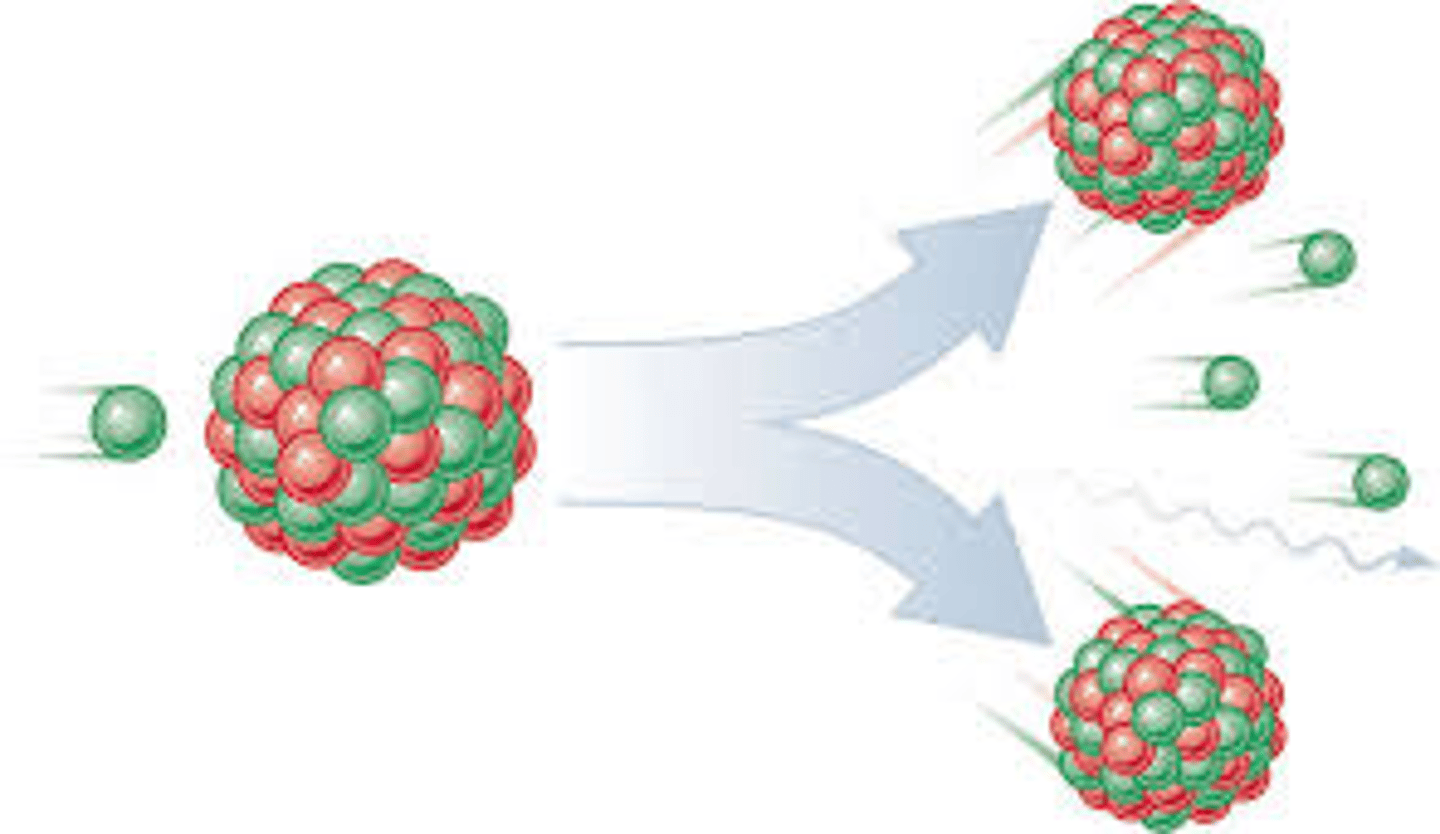
fission
a nuclear reaction in which a massive nucleus splits into smaller nuclei with the simultaneous release of energy
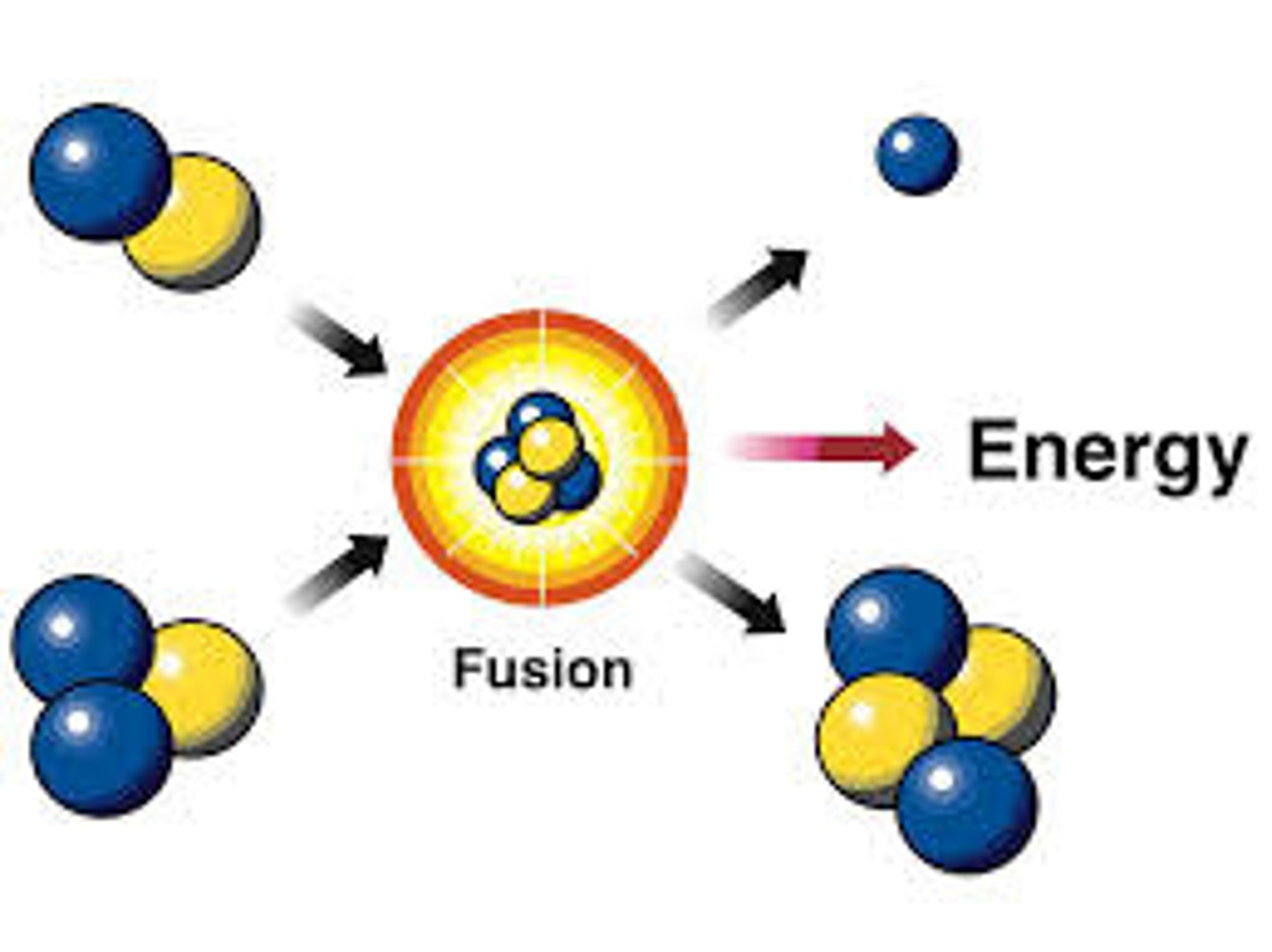
fusion
a nuclear reaction in which nuclei combine to form more massive nuclei with the simultaneous release of energy
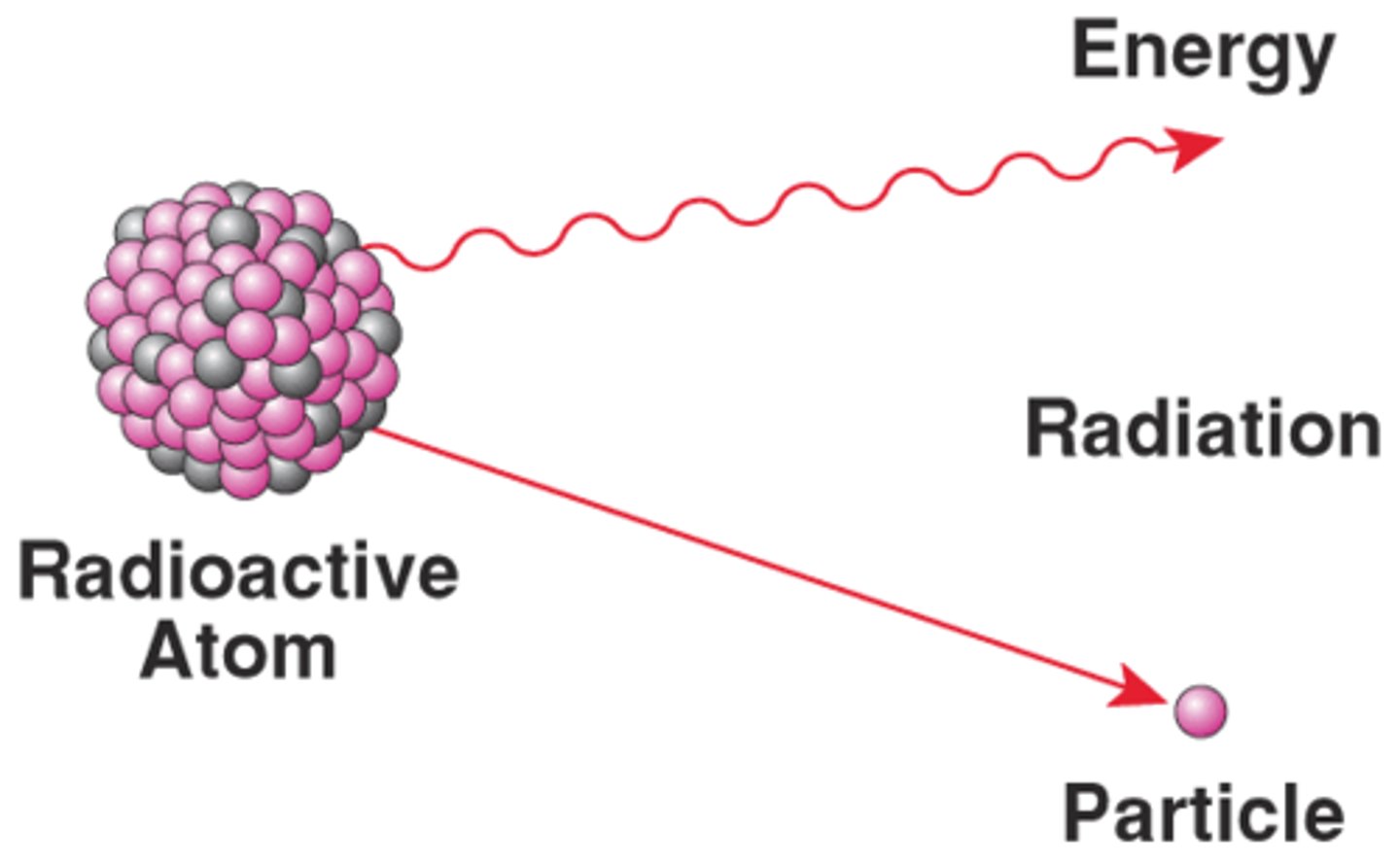
radioactivity
the process by which the nucleus of an atom of an element releases energy and particles
radioactive decay occurs when ______, radioactive atoms give off their energy to become more ______.
unstable; stable
what part of the atom emits energy?
nucleus
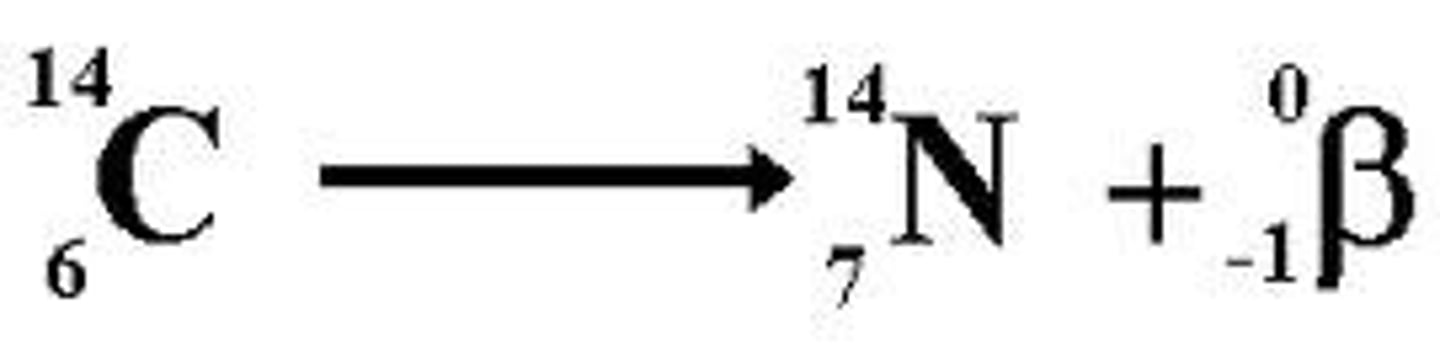
beta decay
emits electron; no change in mass; protons increase 1; neutrons decrease 1
gamma
pure energy; given off in most types of radioactive decay; no change in mass