StemUp: AQA A level Physics 3.8.1 Radioactivity
1/214
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
215 Terms
What did Democritus state about matter in ancient Greece? (1)
Democritus stated that all matter was made out of small, indivisible particles called atomos.
What atomic theory did John Dalton propose in 1804? (2)
- John Dalton proposed that each element is made of a different type of atom.
- These are seen as tiny solid spheres.
What major discovery did JJ Thomson make in 1897? (1)
JJ Thomson discovered the electron and showed that atoms could be broken down into smaller parts.
What was the plum pudding model of the atom? (2)
- The plum pudding model suggested that atoms were spheres of positive charge.
- They also had embedded negatively charged electrons.
What does the plum pudding model look like? (2)
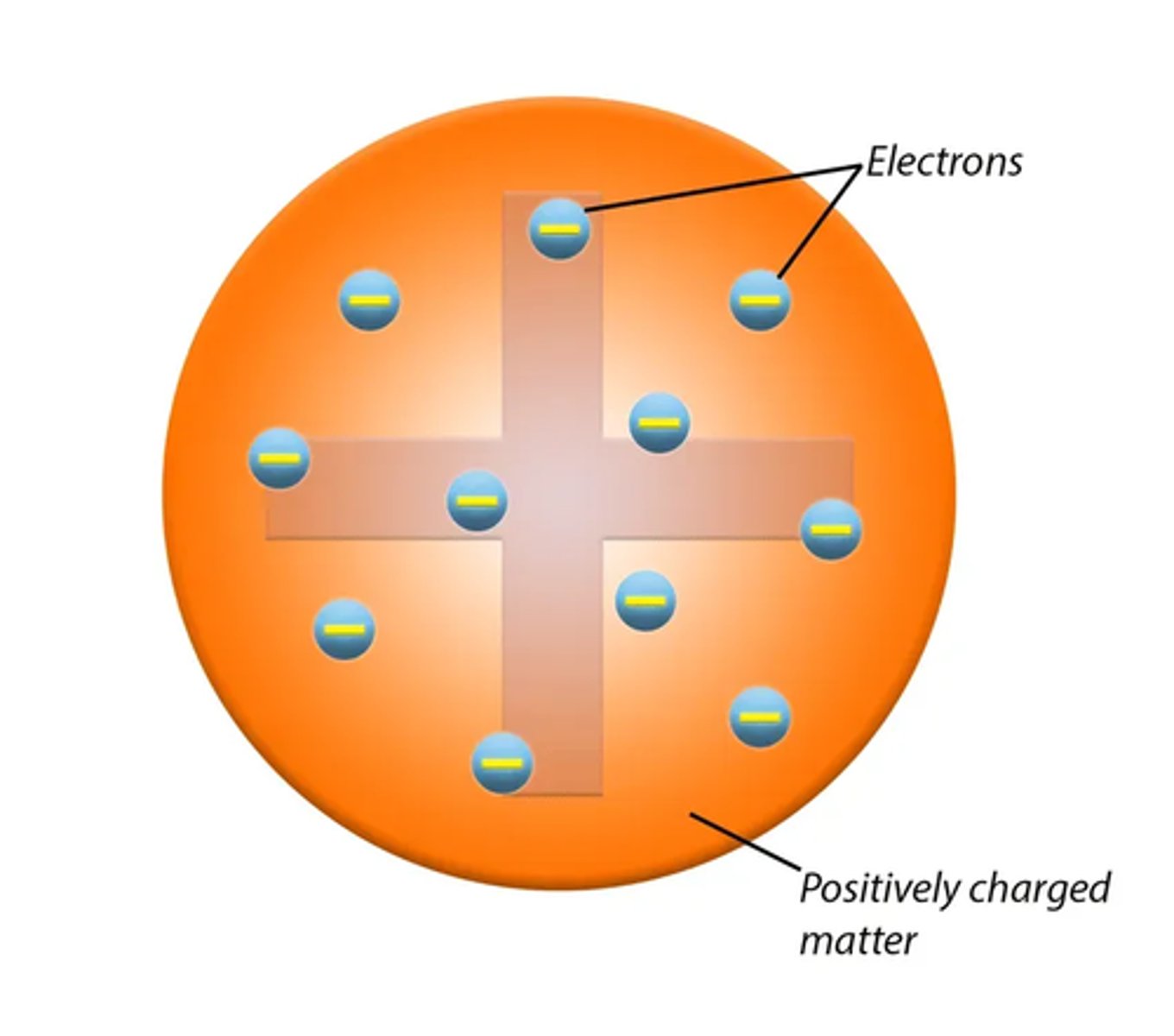
What did Thomson's plum pudding model imply about charge distribution in the atom? (2)
- The plum pudding model implied that positive charge was spread out uniformly.
- It also implied that small electrons were scattered inside.
What was the setup used in Rutherford's scattering experiment? (3)
- Alpha particles were fired at thin gold foil in a vacuum chamber.
- A fluorescent screen coated the chamber to reveal alpha particle strikes.
- A movable microscope was used to detect where the particles hit the screen.
What would the plum pudding model predict about alpha particle deflection? (1)
The plum pudding model predicted that most alpha particles would be deflected only slightly due to weak scattering by electrons.
According to the plum pudding model, how rare would large deflections be? (1)
Large-angle deflections would have been extremely rare or nonexistent according to the plum pudding model.
What was the most common observation from Rutherford's experiment? (1)
Most alpha particles passed straight through the gold foil with no deflection.
What unexpected observations did Rutherford's experiment reveal? (2)
- Some alpha particles were deflected by large angles.
- A very small number of alpha particles were deflected backwards, by more than 90°.
What did the straight-line passage of most alpha particles suggest? (1)
The straight-line passage of most alpha particles suggests that atoms are mostly empty space.
What did large-angle deflections of alpha particles suggest? (1)
The large-angle deflections suggest that there is a small region of concentrated positive charge in the atom.
What did backward deflections of alpha particles imply? (2)
- Backward deflections implied that this region of positive charge is very dense.
- Therefore it can repel fast-moving alpha particles.
What new atomic model resulted from Rutherford's experiment? (2)
- The nuclear model resulted from Rutherford's experiment.
- Atoms have a small, dense, positively charged nucleus at the centre.
How did Rutherford's results disprove the plum pudding model? (1)
The observed deflections contradicted the idea of a diffuse, evenly distributed positive charge.
What is alpha (α) radiation made of? (1)
Alpha radiation is a helium nucleus consisting of 2 protons and 2 neutrons.
What is the relative charge and mass of alpha radiation? (2)
- Alpha particles have a relative charge of +2.
- They have a relative mass of 4.
What is beta minus (β-) radiation made of? (1)
Beta minus radiation is an electron.
What is the relative charge and mass of beta minus radiation? (2)
- Beta minus radiation has a relative charge of -1.
- It has a negligible mass.
What is the relative charge and mass of beta plus radiation? (2)
- Beta plus radiation has a relative charge of +1.
- It has a negligible mass.
What is gamma (γ) radiation? (1)
Gamma radiation is an electromagnetic wave in the gamma region of the spectrum.
What is the charge and mass of gamma radiation? (2)
- Gamma rays have zero charge.
- They have zero mass.
What is the range in air of alpha radiation? (1)
Alpha radiation has a range of 2-10 cm in air.
What is the ionising power of alpha radiation? (1)
Alpha particles are highly ionising.
Is alpha radiation deflected by electric and magnetic fields? (1)
Yes, alpha radiation is deflected by electric and magnetic fields.
What material stops alpha radiation? (1)
Alpha particles are absorbed by paper.
What is the range in air of beta minus radiation? (1)
Beta minus radiation has a range of around 1 metre.
What is the ionising power of beta minus radiation? (1)
Beta minus particles are weakly ionising.
Is beta minus radiation deflected by electric and magnetic fields? (1)
Yes, beta minus is deflected by electric and magnetic fields.
What material is needed to stop beta minus radiation? (1)
Around 3 mm of aluminium is needed to absorb beta radiation.
What is the behaviour of beta plus radiation after emission? (1)
Beta plus radiation is annihilated almost immediately after emission upon encountering an electron.
What is the range in air of beta plus radiation? (1)
Beta plus radiation has effectively has zero range due to immediate annihilation.
What is the range of gamma radiation? (2)
- Gamma radiation has an infinite range.
- It follows the inverse square law.
What is the ionising power of gamma radiation? (1)
Gamma radiation is very weakly ionising.
Is gamma radiation deflected by electric or magnetic fields? (1)
No, gamma rays are not deflected by fields.
What can absorb gamma radiation? (1)
Several inches of lead or metres of concrete are needed to absorb gamma rays.
How is a Geiger-Müller (GM) tube used in absorption tests for identifying radiation types? (1)
A GM tube is used to measure the count rate of a radioactive source during absorption tests.
What is the first step in identifying radiation using absorption tests? (1)
Measure the background count rate with the GM tube before placing the source near it.
How do you test for alpha radiation using absorption materials? (2)
- Place the source near the GM tube and then insert paper.
- If the count rate drops significantly, alpha radiation is present.
How do you test for beta radiation using absorption materials? (2)
- Place the source near the GM tube and then insert aluminium.
- If the count rate drops with aluminium, beta radiation is present (although gamma may also be present if the reduction is not complete).
How do you test for gamma radiation using absorption materials? (2)
- Insert lead between the source and the GM tube.
- If the count rate only drops significantly with lead, gamma radiation is present.
How is beta radiation setup to monitor aluminium foil thickness? (1)
A beta source is placed on one side of the foil and a detector on the other.
What happens if aluminium foil is too thick during production? (2)
- If aluminium foil is too thick, fewer beta particles pass through.
- The detector reading drops, and rollers move closer together.
What happens if aluminium foil is too thin during production? (2)
- If aluminium foil is too thin, more beta radiation is detected.
- The reading of the detector increases, and rollers move further apart.
How are alpha and gamma radiation used to monitor thickness? (1)
Alpha is used for paper, and gamma is used for steel sheets during production.
Why is gamma radiation preferred in medicine? (2)
- Gamma radiation is very weakly ionising.
- It causes less tissue damage than alpha or beta radiation.
How is gamma radiation used as a detector in medicine? (1)
Gamma-emitting tracers with short half-lives are used with gamma cameras to diagnose patients.
How is gamma radiation used to sterilise equipment? (1)
Gamma radiation kills bacteria present on surgical instruments.
How is gamma radiation used in cancer treatment? (2)
- Gamma radiation targets and destroys tumour cells.
- A rotating beam can be used to minimise damage to healthy tissue.
What side effects can result from gamma radiation therapy? (2)
- Gamma radiation therapy can result in short term effects such as fatigue.
- It can also have long-term effects such as infertility.
What is the inverse square law for gamma radiation intensity? (2)
- I = k / x².
- Where I is intensity (arbritary units), k is a constant, and x is the distance from the source (m).
What does the inverse square law tell us about gamma radiation? (1)
Radiation spreads out and decreases with the square of the distance from the source.
How can the inverse square law for gamma radiation be tested? (2)
- Measure count rate (and the corrected count rate) at various distances using a GM tube.
- A plot of corrected count rate against 1 / x² gives a straight line.
How do you correct gamma count rate measurements? (1)
To correct gamma count rate, subtract background count from each measurement.
Why is alpha radiation considered most dangerous internally? (1)
Alpha radiation is highly ionising and causes serious damage if inhaled or ingested.
What is the ionising effect of beta radiation? (1)
Beta radiation is less ionising than alpha radiation but still damages body tissue.
What are the dangers of long exposure to gamma radiation? (1)
Long exposure to gamma radiation can cause cell mutations and damage to healthy tissues.
What tool should be used to move radioactive sources? (1)
Long-handled tongs should be used to move radioactive sources.
How should radioactive sources be stored safely? (1)
Radioactive sources should be stored in a lead-lined container.
How should distance be managed when handling sources? (1)
Keep the source far away from the body to reduce exposure.
What is a key safety rule when pointing a radioactive source? (1)
Never point a radioactive source towards yourself or others.
Why is background radiation important in experiments? (1)
Background radiation is always present from natural and artificial sources.
How is background radiation accounted for in readings? (2)
- Measure background radiation before using the source.
- Then, subtract it from the total count.
What is the formula for corrected count rate? (1)
Corrected count = Total count rate - Background count.
What are natural sources of background radiation? (3)
- Radon gas from rocks.
- Radioactive isotopes in rocks.
- Cosmic rays from space.
What are artificial sources of background radiation? (1)
Nuclear fallout is an artificial source of background radiation.
Why does radioactive decay occur in an unstable nucleus? (1)
An unstable nucleus breaks down to become more stable by releasing particles or energy.
What causes a nucleus to be unstable? (3)
- A nucleus is unstable if it has too many neutrons.
- If it does not have enough neutrons.
- If it has too much energy.
Why is radioactive emission described as ionising radiation? (2)
- Radioactive emission knocks off electrons from atoms.
- This affects outer electrons only, not the nucleus.
What does it mean when we say radioactive decay is random? (1)
It is impossible to predict exactly when a particular nucleus will decay.
What is meant by a constant decay probability? (1)
Constant decay probability means that each nucleus has a fixed probability of decaying per unit time.
What pattern is observed in large samples of radioactive nuclei? (1)
In large samples of radioactive nuclei, the same proportion of nuclei decay in a given time interval.
What is the decay constant? (1)
The decay constant is the probability per second that a nucleus will decay.
What is the defining equation for the decay constant? (2)
- The decay constant is given by ΔN / Δt = -λN.
- Where ΔN is the change in number of nuclei, Δt is time (s), λ is the decay constant (s⁻¹), and N is the number of undecayed nuclei.
What is the exponential decay formula for radioactive nuclei? (2)
- The exponential decay formula for radioactive nuclei is given by N = N₀ × e^(-λt).
- Where N is the number of undecayed nuclei, N₀ is the initial number, λ is the decay constant (s⁻¹), and t is time (s).
How does exponential decay relate to mass? (2)
- Mass is proportional to the number of nuclei.
- So m = m₀ × e^(-λt) can also be used.
How is the exponential decay formula for radioactive nuclei used to find mass in the past? (2)
- Rearrange N = N₀ × e^(-λt) to get N/N₀.
- Then multiply this ratio by current mass to find the mass before a time t has passed.
What is the shape of the graph of nuclei number against time? (2)
- The graph of nuclei number against time shows exponential decay.
- Rapid decrease at first, then slowing, never reaching zero.
What does a graph of nuclei number against time look like? (2)
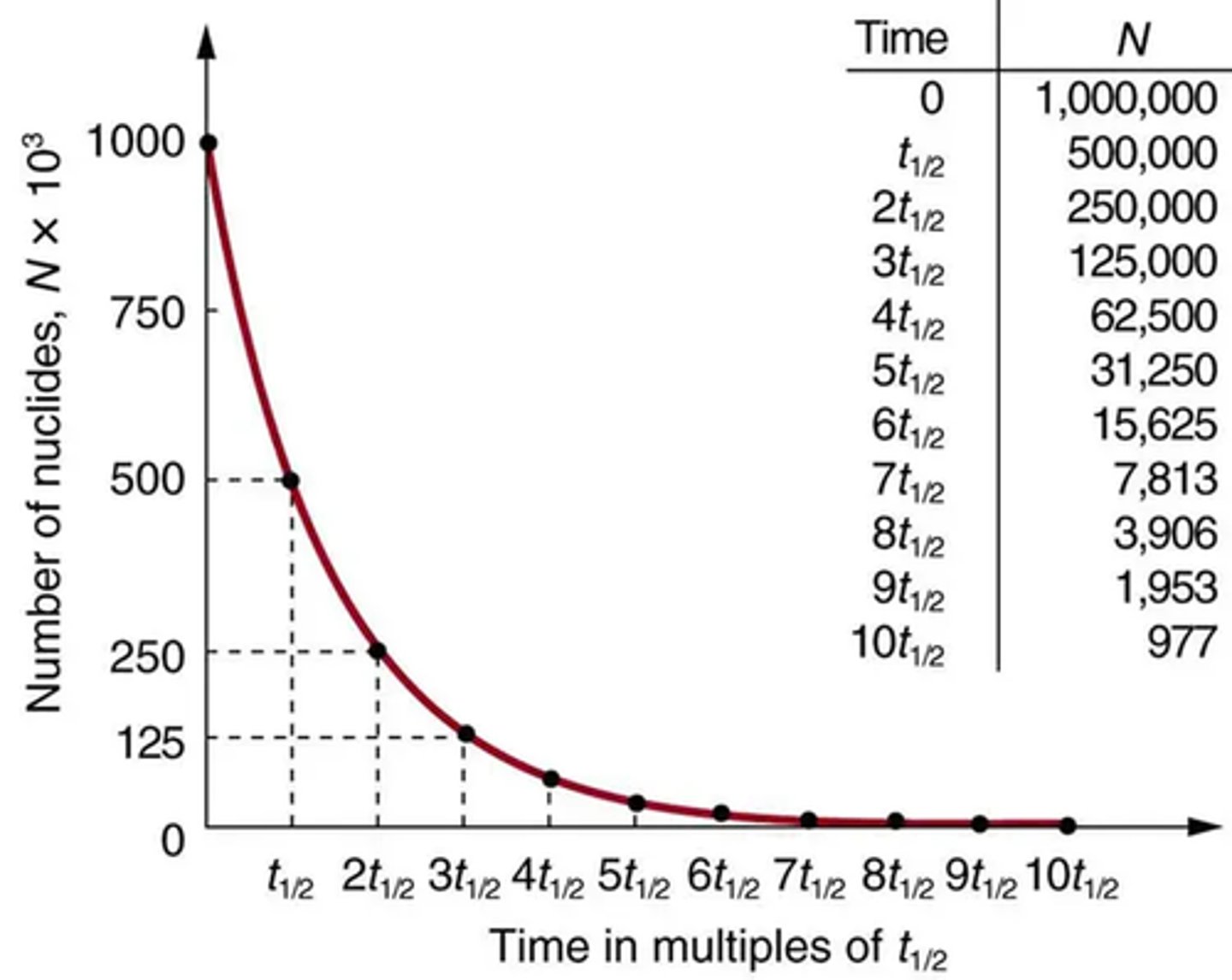
What is half-life? (1)
Half-life is the average time taken for half of the unstable nuclei to decay.
How can you determine half-life from a decay curve? (2)
- Find the time it takes for nuclei number to halve from any point.
- Repeat this to find an average for accuracy.
How do you find half-life from a graph of ln (N) vs. time? (6)
- From N = N₀ × e^(-λt) take the natural log of both sides so ln(N) = ln(N₀ × e^(-λt)).
- Using the log law ln(A*B) = ln(A) + ln(B) this becomes ln(N) = ln(N₀) + ln (e^(-λt)).
- Using the log law ln(e^A) = A this becomes ln(N) = ln(N) = ln(N₀) - λt.
- ln(N) = ln(N₀) - λt, is in the form y = mx + c.
- Plot ln(N) against t from N = N₀ × e^(-λt).
- Giving a straight line with gradient -λ.
What does a graph of ln(N) against time look like? (2)
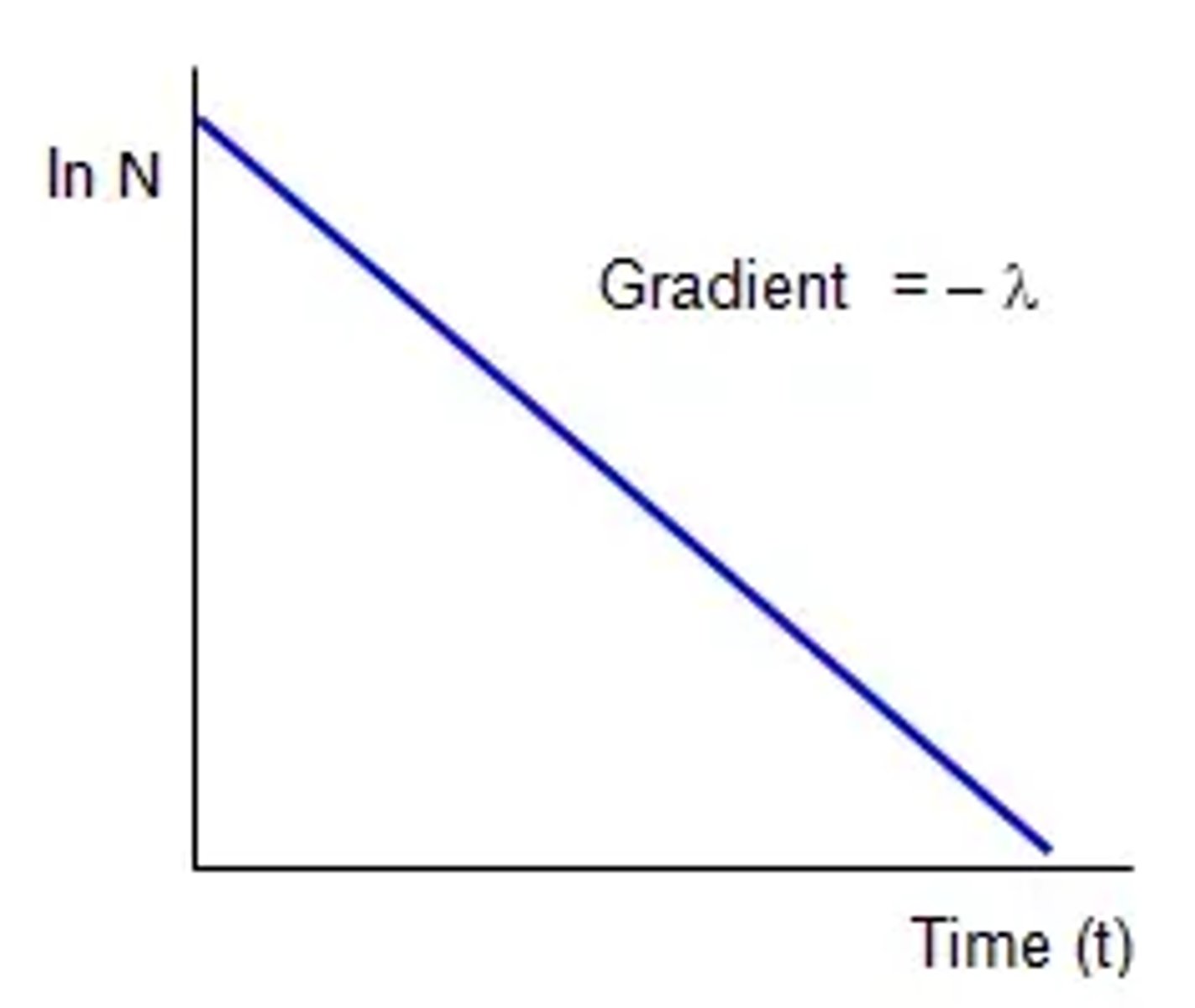
What is the equation linking half-life and decay constant? (2)
- The equation is T₁/₂ = ln(2) / λ.
- Where T₁/₂ is the half-life (s) and λ is the decay constant (s⁻¹).
What is the definition of activity in radioactive decay? (1)
Activity is the number of nuclei that decay per second.
What is the unit of activity? (2)
- Becquerel (Bq) is the unit of activity.
- Where 1 Bq = 1 decay per second.
What is the equation for activity? (2)
- The equation for activity is given by A = λN.
- Where A is the activity (Bq), λ is the decay constant (s⁻¹), and N is number of undecayed nuclei.
What is the exponential decay equation for activity? (2)
- The exponential decay equation for activity is given by A = A₀ × e^(-λt).
- Where A is the activity at time t (Bq), A₀ is the initial activity (Bq), λ is the decay constant (s⁻¹), and t is time (s).
Why is the time for activity to halve equal to half-life? (2)
- Activity is directly proportional to the number of undecayed nuclei.
- So the time the nuclei takes to halve (half-life) is equal to the time for activity to halve.
When is the decay constant valid to use in models? (1)
The decay constant should only be used in models when the sample contains a large number of nuclei.
Why is a large sample needed for decay constant modelling? (1)
Radioactive decay is random and statistical predictions require large numbers.
What is a use of a long half-life isotope? (1)
Carbon-14 (T₁/₂ = 5730 years) is used to date organic archaeological samples.
What is a use of a short half-life isotope? (1)
Technetium-99m (T₁/₂ = 6 hours) is used for medical gamma imaging.
Why is technetium-99m suitable for diagnostics? (2)
- Technetium-99m has a short half-life which limits exposure.
- Gamma rays allow internal imaging.
Why are isotopes with long half-lives stored securely? (1)
Isotopes with long half-lives remain radioactive for hundreds of years.
How are long-lived isotopes stored safely? (1)
Long-lived isotopes are stored in underground steel casks to prevent environmental contamination.
What does the atomic structure notation show? (2)
- The atomic structure notation shows the element's symbol, with nucleon number on the top left.
- The proton number is shown on the bottom left.
What does the atomic structure notation look like? (2)
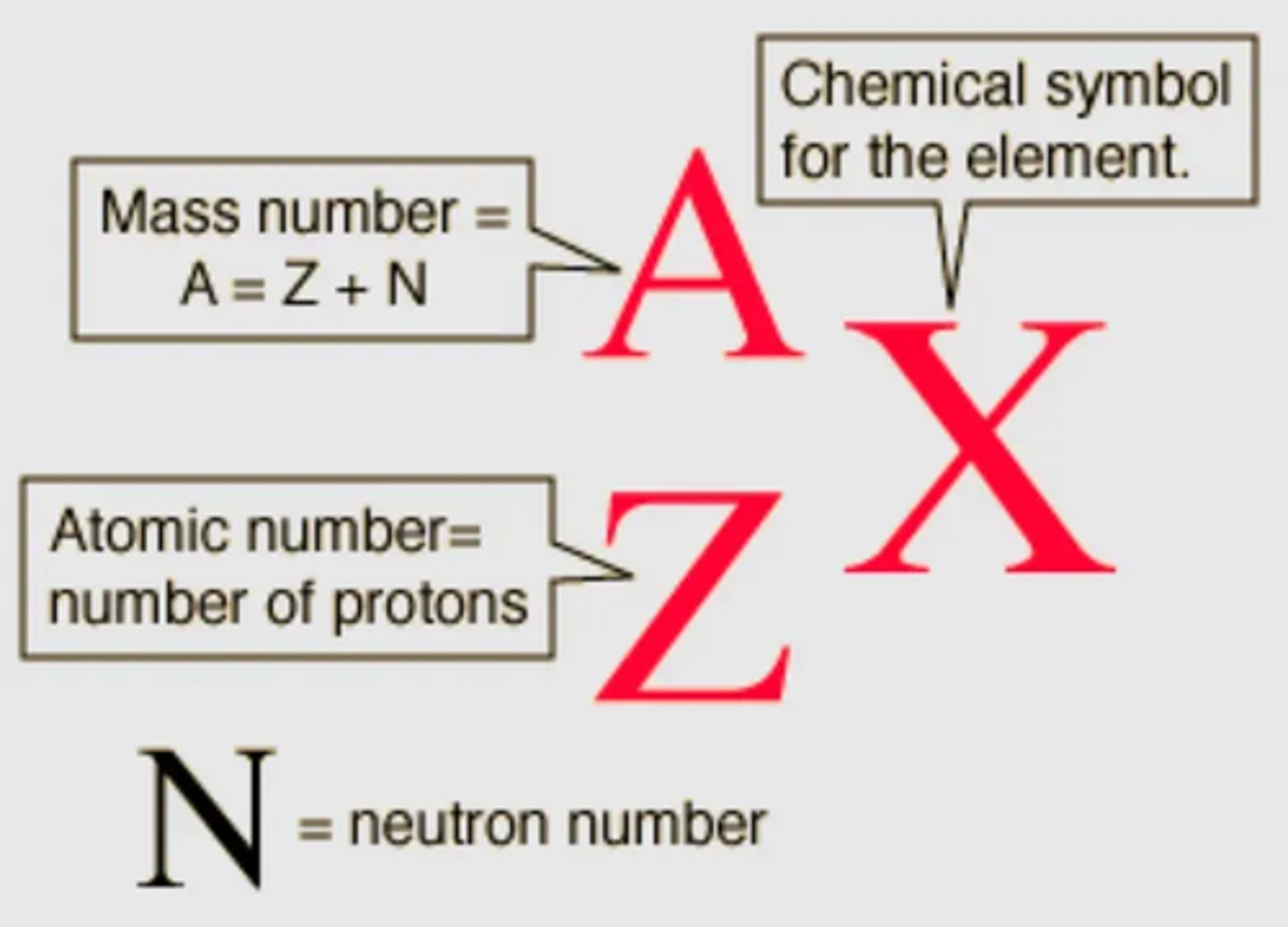
What does the neutron vs atomic number graph show? (2)
- The neutron vs atomic number graph shows the band of stability, as stable nuclei lie close to a line.
- More neutrons are needed at higher atomic numbers to keep stability.