Ch10 Stuff
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
111 Terms
Why are there so many organic compounds?
carbon form stable, covalent bonds with other carbon atoms. carbon can form up to 4 covalent bonds with other carbon
Carbon atoms form stable bonds with other elements, such as:
oxygen, nitrogen, sulfur, halogen, the presence of these other elements confers many new physical and chemical properties on organic compound
Carbon atoms form double or triple bonds with other atoms to produce
a variety of structures with differing properties
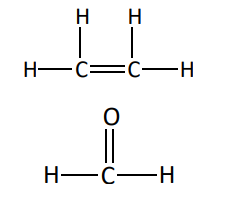
What kind of bonds are these
double bonds
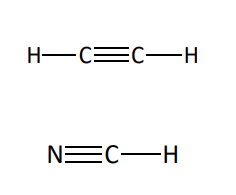
What kind of bonds are these
triple bonds
What are some ways to arrange carbon and other atoms?
branched chains, ring structures, and linear chains
Differences between organic and inorganic compounds:
Bond type
organics have covalent bonds (electron sharing), inorganics usually have ionic bonds (electron transfer)
Differences between organic and inorganic compounds:
structure
Organic: molecules, nonelectrolytes. Inorganics: three dimensional crystal structures, often water soluble, dissociating into ions-electrolytes
Differences between organic and inorganic compounds:
Physical Differences, Melting Point and Boiling Point
organics have lower melting points, intermolecular forces are broken fairly easily. Inorganics usually have higher melting points, ionic bonds require more energy to break
Differences between organic and inorganic compounds:
Physical Differences, Water solubility
organics are nonpolar, water insoluble. Inorganics are water-soluble, readily dissociate
What do hydrocarbons contain
only carbon and hydrogen
What kind of molecules are hydrocarbons
nonpolar, not soluble in water
What are hydrocarbons soluble in
typical nonpolar organic solvents, ex: toluene and pentane
What are hydrocarbons constructed of
chains or rings of carbon atoms with sufficient hydrogen atoms to fulfill carbons need for four bonds
What do substituted hydrocarbons have
at least one hydrogen atom that is replaced by another atom or group of atoms
contain only single bonds, for example: ethane, CH3CH3
alkanes
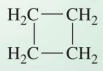
Alkanes with carbon atoms bonded in rings
cycloalkanes
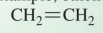
Contain at least one double bond, for example, ethene
alkenes
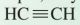
contain at least one triple bond, for example, ethyne
alkynes
Alkanes are
compounds that contain only carbon-carbon and carbon-hydrogen single bonds
A saturated hydrocarbon has no
double or triple bonds
Alkenes and alkynes unsaturated hydrocarbons because
they contain at least one carbon to carbon double or triple bond
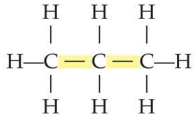
saturated hydrocabron
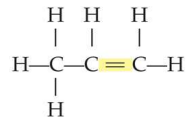
unsaturated hydrocarbon
Cyclic structure of hydrocarbons
form a closed ring
Aromatic hydrocarbons contain a
benzene ring or related structure
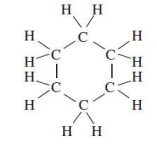
cycloalkane/cyclohexane
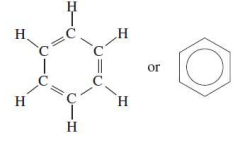
an aromatic hydrocarbon/benzene
general formula for a chain alkane is
CnH2n+2
CnH2n+2
What is n
the number of carbon atoms in the molecule
Are alkanes saturated hydrocarbons
yes, they contain only carbon and hydrogen. Bonds are carbon-hydrogen and carbon-carbon single bonds
Molecular formula
C2H6 , C3H8
lists kind and number of each type of atom in a molecule, no bonding pattern
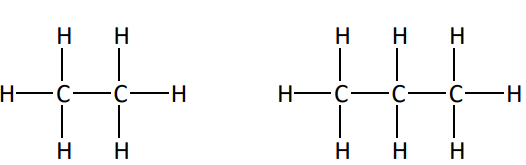
Structural formula
shows each atom and bond in a molecule
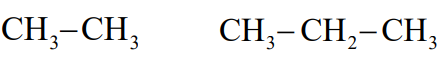
Condensed formula
shows all the atoms in a molecule in sequential order indicating which atoms are bonded to which

Skeletal Structure
assumes a carbon atom at any location where lines intersect, each carbon in the structure is bonded to the correct number of hydrogen atoms
Alkanes molecular formula
CnH2n+2
Methane molecular formula
CH4
Ethane molecular ethane
C2H6
Propane molecular formula
C3H8
Butane molecular formula
C4H10
Pentane molecular formula
C5H12
Hexane molecular formula
C6H14
Heptane molecular formula
C7H16
Octane molecular formula
C8H18
Nonane molecular formula
C9H20
Decane molecular formula
C10H22
Methane condensed formula
CH4
Ethane condensed formula
CH3CH3
Propane condensed formula
CH3CH2CH3
Butane condensed formula
CH3CH2CH2CH3 or CH3(CH2)2CH3
Pentane condensed formula
CH3CH2CH2CH2CH3 or CH3(CH2)3CH3
Hexane condensed formula
CH3CH2CH2CH2CH2CH3 or CH3 (CH2 )4CH3
Heptane condensed formula
CH3CH2CH2CH2CH2CH2CH3 or CH3 (CH2 )5CH3
Octane condensed formula
CH3CH2CH2CH2CH2CH2CH2CH3 or CH3 (CH2 )6CH3
Nonane condensed formula
CH3CH2CH2CH2CH2CH2CH2CH2CH3 or CH3 (CH2)7CH3
Decane condensed formula
CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 or CH3(CH2)8CH3
Many carbon compounds exist in the form of
isomers
Isomers are
compounds with the same molecular formula but different structurees
Physical properties of hydrocarbons
nonpolar molecules, not water soluble; soluble in nonpolar organic solvents, low melting points and low boiling point, generally less dense (lighter) than water, as length (molecular weight) increases melting and boiling points increase as does the densitity
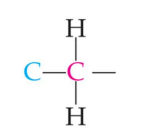
primary carbon
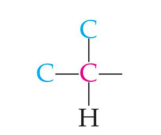
secondary carbon
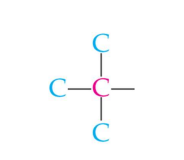
tertiary carbon
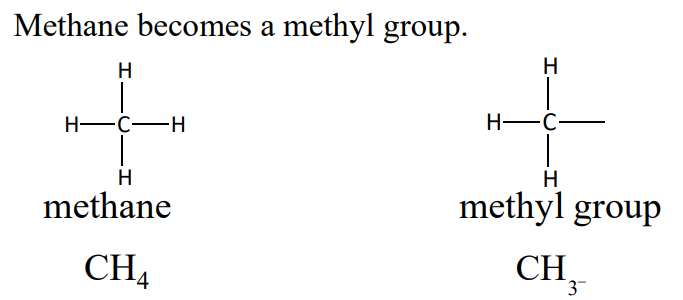
An ___ ___ is an alkane with one hydrogen atom removed. It is named by replacing the -ane of the alkane name with -yl
alkyl group
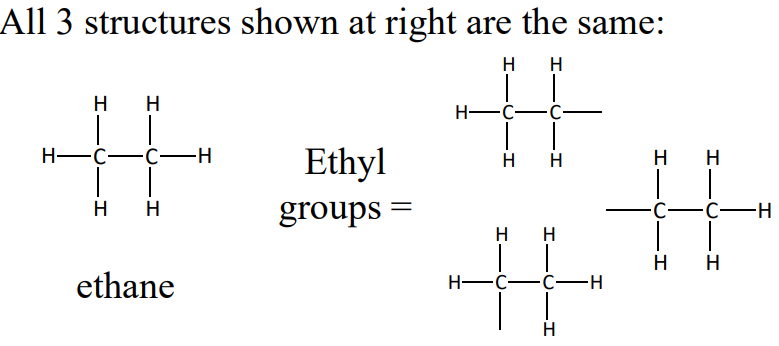
Ethyl groups, using ethane as the example
for ethane, all 6 H’s are equivalent. Removing one H generates the ethyl group
Alkyl Group Structure:
-CH3
Methyl
Alkyl Group Structure:
-CH2CH3
ethyl
Alkyl Group Structure:
-CH2CH2CH3
propyl
Alkyl Group Structure:
-CH2CH2CH2CH3
Butyl
Alkyl Group Structure:
-CH2CH2CH2CH2CH3
Pentyl
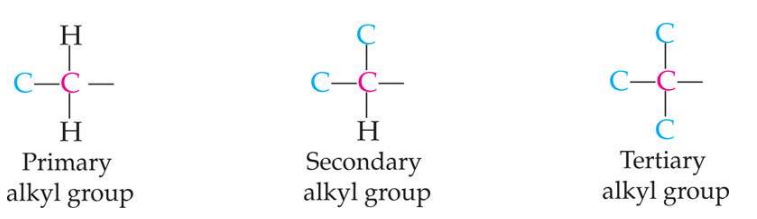
How are alkyl groups classified
According to the number of carbons attached to the carbon atom that joins the alkyl group to a molecule. All continuous chain alkyl groups are 1 degree. Isopropyl and sec-butyl are 2-degree groups
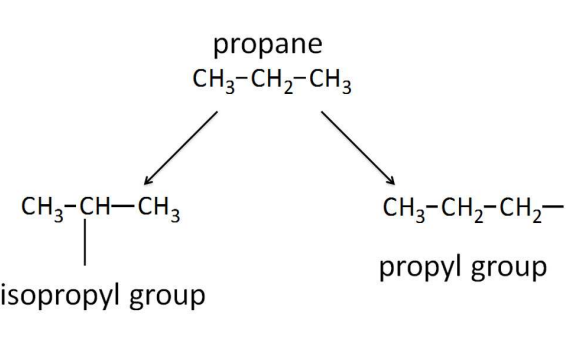
Iso- Alkyl Groups
Propane gives two propyl groups, depending on whether an end (1 degree) or interior (2 degrees) H is removed
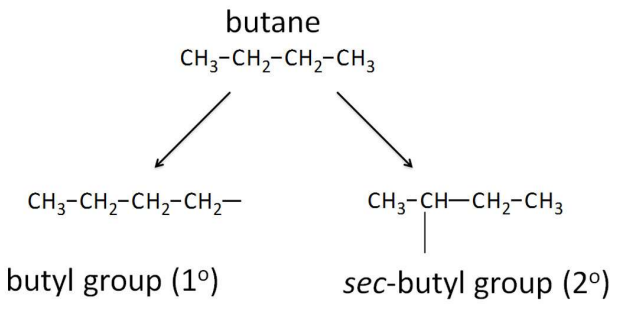
Sec- Alkyl Groups
Butane gives two butyl groups, depending on whether an end (1 degree) or interior (2 degrees) H is removed
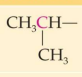
Classification:
Common name:
IUPAC Name:
secondary 2. Isopropyl 3. 1-Methylethyl
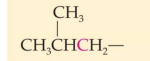
Classification:
Common name:
IUPAC Name:
primary 2. Isobutyl 3. 2-Methylpropyl
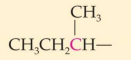
Classification:
Common name:
IUPAC Name:
secondary 2. sec-buytl 3. 1-methylpropyl
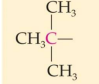
Classification:
Common name:
IUPAC Name:
tertiary 2. t-Butyl or tert-butyl 3. 1,1- Dimethylethyl
IUPAC
international union of pure and applied chemistry
Carbon chain length: 1
prefix and alkane name
meth-, methane
Carbon chain length: 2
prefix and alkane name
eth-, ethane
Carbon chain length: 3
prefix and alkane name
prop-, propane
Carbon chain length: 4
prefix and alkane name
but-, butane
Carbon chain length: 5
prefix and alkane name
pent-, pentane
Carbon chain length: 6
prefix and alkane name
hex-, hexane
Carbon chain length: 7
prefix and alkane name
hept-, heptane
Carbon chain length: 8
prefix and alkane name
oct-, octane
Carbon chain length: 9
prefix and alkane name
non-, nonane
Carbon chain length: 10
prefix and alkane name
dec-, decane
IUPAC Names for Alkanes, Rule One
The base or parent name for an alkane is determined by the longest chain of carbon atoms in the formula. The longest chain may bend and twist. Any carbon groups not part of the base chain are called branches or substituents. These carbon groups are also called alkyl groups
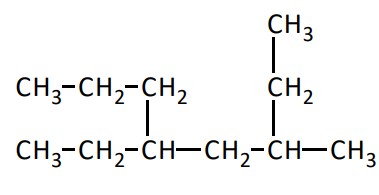
What is the longest chain in the molecule
8 carbon chain, octane
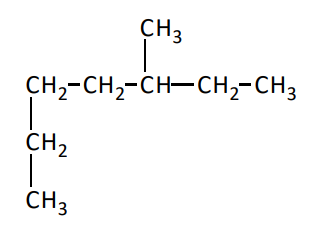
What is the longest chain in the molecule
7 carbon chain, heptane
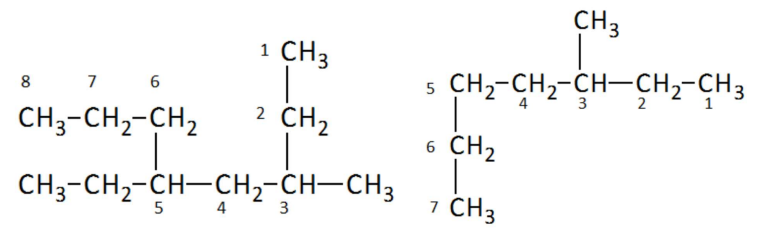
IUPAC Names for Alkanes, Rule 2
Number the carbon atoms in the chain starting from the end with the first branch. If both branches are equally from the ends, continue until a point of difference occurs
IUPAC Names for Alkanes, Rule 3
Write each of the branches/substituents in alphabetical order before the base/stem name (longest chain). Halogens usually come first, indicating the position of the branch on the main chain by prefixing its name with the carbon number to which it is attached. Separate numbers and letters with a hyphen. Separate two or more numbers with commas.
When naming alkanes, what do you do when a branch/substituent occurs more than once
Prefix the name with di, tri, or tetra, then list the number of the carbon branch for that substituent to the name with a separate number for each occurrence. Separate numbers with commas
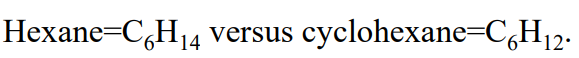
cycloalkanes have
two less hydrogens than the corresponding chain alkane
to name cycloalkanes, prefix clyco- to the name of the corresponding alkane
place substituents in alphabetical order before the base name name as for alkanes. For multiple substituents, use the lowest possible set of numbers, a single substituent requires no number
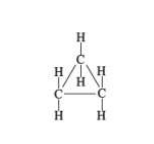
what cycloalkane structure is this
structural formula cyclopropane

what cycloalkane structure is this
cyclopropane line formula
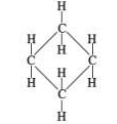
what cycloalkane structure is this
cyclobutane structural formula

what cycloalkane structure is this
cyclobutane line formula
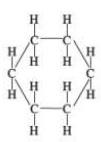
what cycloalkane structure is this
cyclohexane structural formula