Chem Honors - Common Elements, Ions, and Periodic Table Quiz, Covalent Compounds, and Acids
1/132
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
133 Terms
What does the chemical symbol Al stand for?
Aluminum
What does the chemical symbol Ar stand for?
Argon
What does the chemical symbol As stand for?
Arsenic
What does the chemical symbol Ba stand for?
Barium
What does the chemical symbol B stand for?
Boron
What does the chemical symbol Br stand for?
Bromine
What does the chemical symbol Cd stand for?
Cadmium
What does the chemical symbol Ca stand for?
Calcium
What does the chemical symbol C stand for?
Carbon
What does the chemical symbol Cl stand for?
Chlorine
What does the chemical symbol Cr stand for?
Chromium
What does the chemical symbol Co stand for?
Colbat
What does the chemical symbol Cu stand for?
Copper
What does the chemical symbol F stand for?
Fluorine
What does the chemical symbol Au stand for?
Gold
What does the chemical symbol He stand for?
Helium
What does the chemical symbol H stand for?
Hydrogen
What does the chemical symbol I stand for?
Iodine
What does the chemical symbol Fe stand for?
Iron
What does the chemical symbol Pb stand for?
Lead
What does the chemical symbol Li stand for?
Lithium
What does the chemical symbol Mg stand for?
Magnesium
What does the chemical symbol Mn stand for?
Manganese
What does the chemical symbol Hg stand for?
Mercury
What does the chemical symbol Ne stand for?
Neon
What does the chemical symbol Ni stand for?
Nickel
What does the chemical symbol N stand for?
Nitrogen
What does the chemical symbol O stand for?
Oxygen
What does the chemical symbol P stand for?
Phosphorus
What does the chemical symbol Pt stand for?
Platinum
What does the chemical symbol K stand for?
Potassium
What does the chemical symbol Ra stand for?
Radium
What does the chemical symbol Si stand for?
Silicon
What does the chemical symbol Ag stand for?
Silver
What does the chemical symbol Na stand for?
Sodium
What does the chemical symbol Sr stand for?
Strontium
What does the chemical symbol S stand for?
Sulfur
What does the chemical symbol Sn stand for?
Tin
What does the chemical symbol Ti stand for?
Titanium
What does the chemical symbol U stand for?
Uranium
What does the chemical symbol Zn stand for?
Zinc
Do the protons ever change?
No electrons only change
If there are equal electrons and protons what is the charge?
No charge
What is a cation?
Positive ion that lost an electron
What is a anion?
A negative ion that gained an electron - can be more than one
What happens if an element has roman numerals?
It is always a cation with less electrons
When finding the amount of protons and neutrons, if the atomic mass is odd what do you do?
You would subtract the amount of protons from the atomic mass
The protons are the atomic number
Are the atomic number and amount of protons and electrons an element has the same?
Yes only if it is neutral
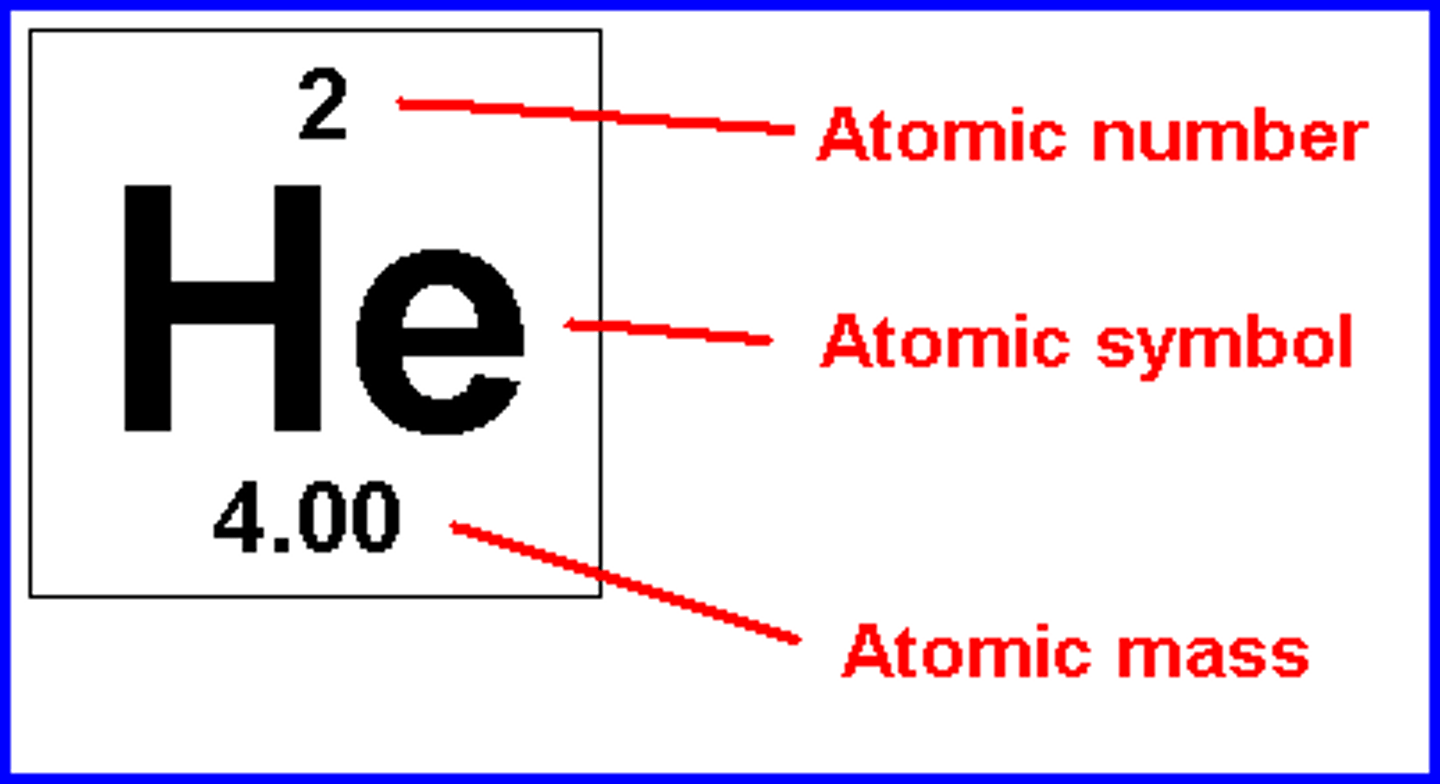
Which elements don't have roman numerals?
Silver, Zinc, and Cadmium
What are elements with +2 charge?
Cadmium, Chromium (II), Cobalt(II), Copper(II), Iron(II), Lead (II), Manganese(II), Mercury (I), Mercury(II), Nickel(II), and Zinc
What are common elements with a +3 charge?
Chromium(III), Cobalt(III), Iron(III), Manganese(III), Nickel(III)
What are common elements with a +4 charge?
Lead(IV) and Tin(IV)
What are common elements with a +1 charge?
Silver and Copper(I)
What are common polyatomic ions with a +1 charge?
NH₄+¹
What are common polyatomic ions with a -1 charge?
C₂H₃O₂-¹
HCO₃-¹
CN-¹
OH-¹
CIO-¹
CIO₂-¹
CIO₃-¹
CIO₄-¹
MnO₄-¹
NO₂-¹
NO₃-¹
H₂PO₄-¹
What are common polyatomic ions with a -2 charge?
CO₃-²
CrO₄-²
Cr₂O₇-²
HPO₄-²
C₂O₄-²
SiO₃-²
SO₃-²
SO₄-²
What are common polyatomic ions with a -3 charge?
PO₃-³
PO₄-³
What does NH₄+¹ stand for?
Ammonium
What does C₂H₃O₂-¹ stand for?
Acetate
What does HCO₃-¹ stand for?
Bicarbonate
What does CN-¹ stand for?
Cyanide
What does OH-¹ stand for?
Hydroxide
What does CIO-¹ stand for?
Hypochlorite
What does CIO₂-¹ stand for?
Chlorite
What does CIO₃-¹ stand for?
Chlorate
What does CIO₄-¹ stand for?
Perchlorate
What does MnO₄-¹ stand for?
Permanganate
What does NO₂-¹ stand for?
Nitrite
What does NO₃-¹ stand for?
Nitrate
What does H₂PO₄-¹ stand for?
Dihydrogen Phosphate
What does CO₃-² stand for?
Carbonate
What does CrO₄-² stand for?
Chromate
What does Cr₂O₇-² stand for?
Dichromate
What does HPO₄-² stand for?
Hydrogen phosphate
What does C₂O₄-² stand for?
Oxalate
What does SiO₃-² stand for?
Silicate
What does SO₃-² stand for?
Sulfite
What does SO₄ stand for?
Sulfate
What does PO₃-³ stand for?
Phosphite
What does PO₄-³ stand for?
Phosphate
What does it mean when a polyatomic ion starts with per?
Per- means the ion has the most oxygen possible in that family
What does it mean when a polyatomic ion starts with hypo?
Hypo- means the least oxygens
What does it mean when a polyatomic ion starts with bi?
Bi- means a hydrogen is added and the charge becomes one more positive
If there is one of the last element in the compound what would the name begin with?
Mono-
If there is two of the last element in the compound what would the name begin with?
Di-
If there is three of the last element in the compound what would the name begin with?
Tri-
If there is four of the last element in the compound what would the name begin with?
Tetra-
If there is five of the last element in the compound what would the name begin with?
Penta-
If there is six of the last element in the compound what would the name begin with?
Hexa-
If there is seven of the last element in the compound what would the name begin with?
Hepta-
If there is eight of the last element in the compound what would the name begin with?
Octa-
If there is nine of the last element in the compound what would the name begin with?
Nona-
If there is ten of the last element in the compound what would the name begin with?
Deca-
How do you name covalent compounds?
The less electronegative element is written 1st
It is given a prefix only if it has more than one atom
The second element is named by combining (a) prefix, (b) root name, and (c) –ide ending
What is the ending for covalent compounds?
-ide
What happens if the last element in the covalent compound ends in a vowel?
The O or a at the end of a prefix is usually dropped when the next letter is a vowel. (i.e. monoxide/pentoxide)
What is an acid?
An Acid is a compound that donates a proton (H+) in solution
What does acids always contain?
Hydrogen
What are oxyacids?
Many common acids contain only oxygen, hydrogen, and a non-metallic ion or polyatomic ion - OXYACIDS
How do you name binary acids?
Binary Acids - 2 parts: hydrogen + element
Naming: "Hydro" + element root + "ic" + acid
EX: HCl - Hydrochloric Acid